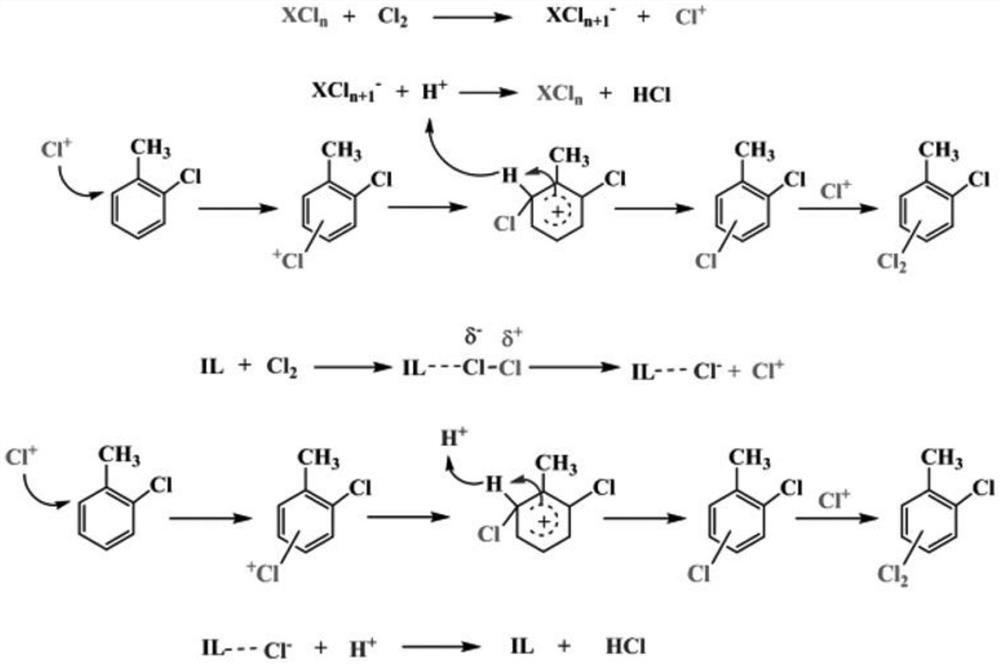
Method for synthesizing 2, 6-dichlorotoluene by directionally chlorinating o-chlorotoluene with novel composite catalyst
A technology of composite catalyst and o-chlorotoluene, applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., can solve the problems of long process flow, many reaction steps, low yield, etc. Simple steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Take a 500ml four-necked flask, add 100g of o-chlorotoluene, weigh 3g of anhydrous aluminum chloride and 1g of triethylamine under inert gas conditions, respectively, weigh the 3g of anhydrous aluminum chloride and 1g of the above-mentioned steps. Triethylamine was added to the above-mentioned four-necked flasks respectively; the four-necked flasks were placed in an oil bath, and heated and stirred for 6h under the condition of passing chlorine gas. After 6 hours of reaction, samples were taken for analysis.
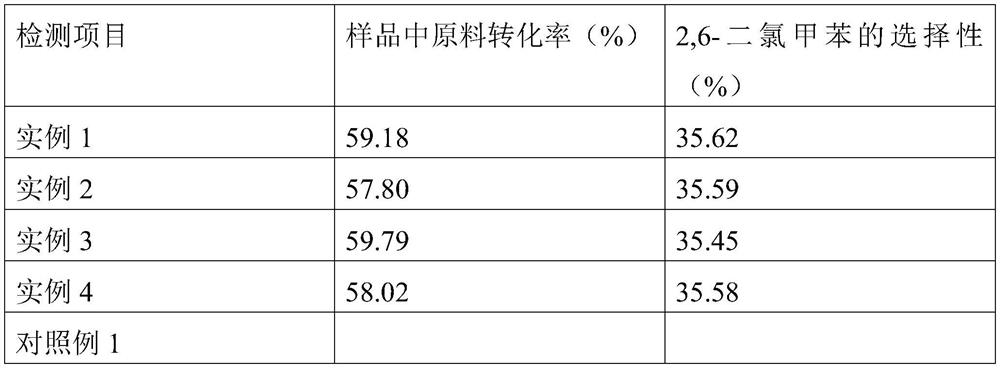
[0031] The conversion of raw materials in the sample was 59.18%, and the selectivity of 2,6-dichlorotoluene was 35.62%.
example 2
[0033] Take a 500mL four-necked flask, add 95g of o-chlorotoluene, weigh 3g of anhydrous aluminum chloride and 1g of triethylamine under inert gas conditions, respectively, weigh 3g of anhydrous aluminum chloride and 1g of the above-mentioned steps. Triethylamine was added to the above-mentioned four-necked flasks respectively; the four-necked flasks were placed in an oil bath, and heated and stirred for 6h under the condition of passing chlorine gas. After 6 hours of reaction, samples were taken for analysis.
[0034] The conversion of raw materials in the sample was 57.80%, and the selectivity of 2,6-dichlorotoluene was 35.59%.
example 3
[0036] Take a 500mL four-necked flask, add 105g of o-chlorotoluene, weigh 3.2g of anhydrous aluminum chloride and 1.1g of triethylamine under inert gas conditions, and weigh 3.2g of anhydrous chlorinated Aluminum and 1.1 g of triethylamine were respectively added to the above-mentioned four-necked flask; the four-necked flask was placed in an oil bath, and heated and stirred for 6.5h under the condition of passing chlorine gas. After 6.5 hours of reaction, samples were taken for analysis.
[0037] The conversion of raw materials in the sample was 59.79%, and the selectivity of 2,6-dichlorotoluene was 35.45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com