Benzimidazole derivative as well as preparation method and application thereof
A technology of benzimidazole and derivatives, applied in the field of benzimidazole derivatives and their preparation, can solve the problems of DNA repair defects, cell cycle arrest, p62 accumulation, etc., achieve significant proliferation and growth, inhibit H2A ubiquitination, Proliferative and growth inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment describes the benzimidazole derivatives and the preparation method thereof. The structural formula of the benzimidazole derivatives is as follows: figure 1 As shown, the preparation method of this benzimidazole derivative is as follows:
[0044]
[0045] Concretely, the preparation method of this benzimidazole derivative is exemplified below:
[0046] 1.56g of compound 1 acid chloride (5.0mmol) and 1.60mL of N,N-diethylaniline (10mmol) were added to 40mL of DCM to prepare a mixed suspension, and 1.04g of compound 2N-Boc was added at -78°C -1,2-phenylenediamine 2 (5.0 mmol) was mixed and incubated for 1 hour, then heated to room temperature, and after stirring for 5 hours, a yellow solid was precipitated from the solvent and filtered to obtain compound 3 in 92% yield;
[0047] At room temperature, 66 mg of compound 3 (0.1 mmol) and compound 4 (0.21 mmol) were stirred and mixed in 3 mL of DCM overnight. After the reaction was completed, the solvent was...
Embodiment 2
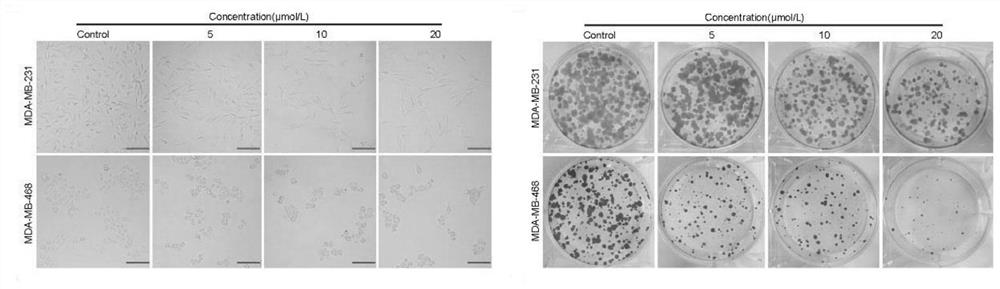
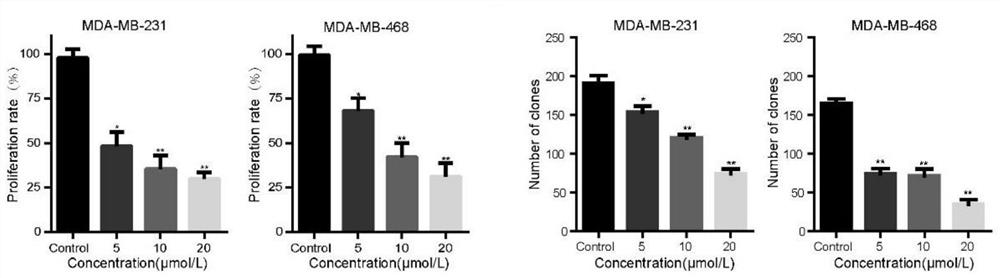
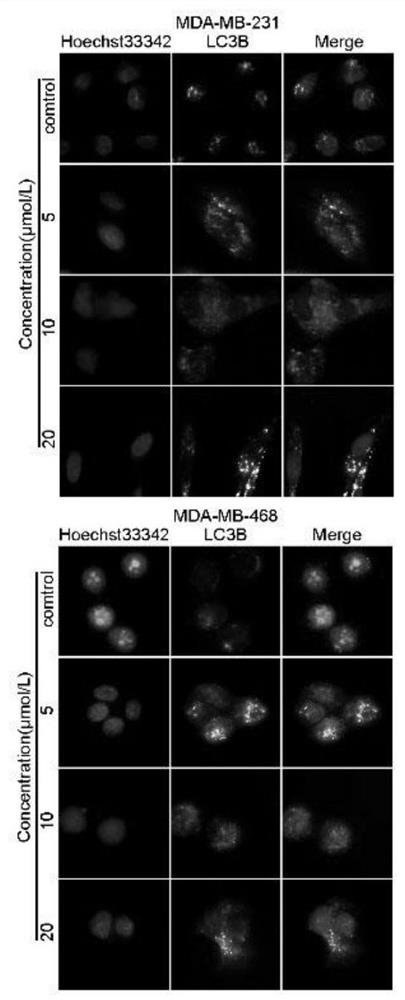
[0053] Example 2 Related Cell Tests
[0054] The human triple negative breast cancer cell lines MDA-MB-231 and MDA-MB-468 used in this example were purchased from the American Type Culture Collection (ATCC, Virginia, USA). These cells were incubated at 37°C with 5% CO. 2 The cells were grown in high glucose DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin / streptomycin (Gibco) in a humidified incubator of
[0055] (1) Cell viability assay
[0056] The antiproliferative activity of the compounds was determined by MTT method, namely, MDA-MB-231 and MDA-MB-468 cells 24, 48 and 24 were treated with 0, 0.83, 1.56, 3.125, 6.25, 12.5, 25, 50 and 100 μmol / L test compounds. 72 hours. Next, 20 μL of MTT solution was added to each well and incubated for an additional 4 hours, then dissolved in 200 μL of DMSO. Absorbance values were measured at 570 nm using a microplate reader (Bio-Tek, VT, USA) and data were analyzed by GraphPad Prism 6. All...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com