Preparation method of natural product veranamine alkaloid
A technology of natural products and alkaloids, applied in the direction of organic chemistry, can solve problems such as low yield and harsh reaction conditions, and achieve the effects of reducing costs, improving safety, and simplifying experimental operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
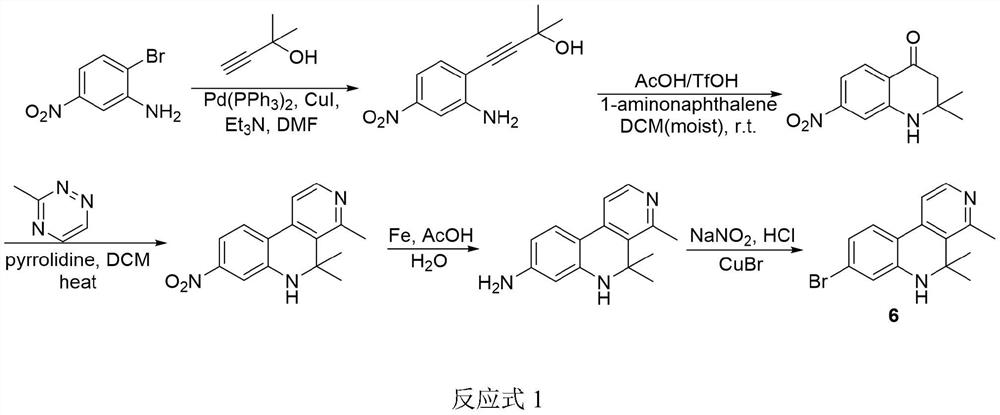
[0052] (5) Preparation of Veranamine: Ethyl 2-acetamido-1-(7-bromo-2,2-dimethyl-1,2-dihydroquinolin-4-yl)acetate Phosphorus-catalyzed ring closure to produce the alkaloid veranamine.
[0053] Reaction 3 is as follows:
[0054]
Embodiment 1
[0055] Example 1: Preparation of Veranamine
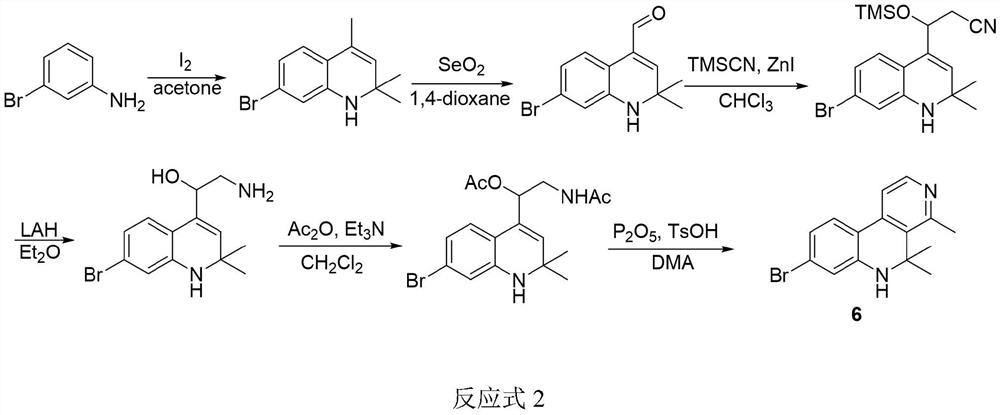
[0056] (1) Preparation of 7-bromo-2,2,4-trimethyl-1,2-dihydroquinoline (2)
[0057] Its reaction formula is:
[0058]
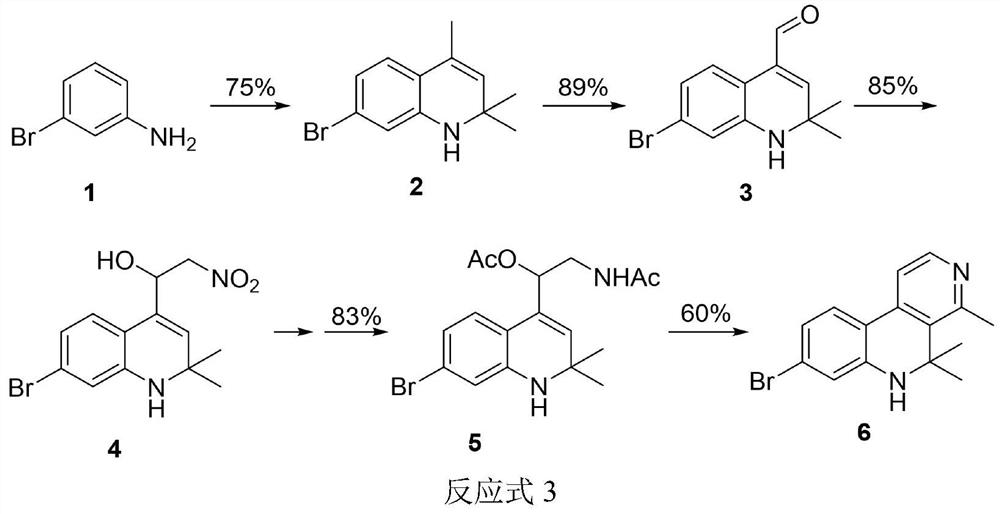
[0059] 3-Bromoaniline (20.014g, 116.34mmol) and iodine (5.906g, 23.27mmol) were dissolved in acetone (300mL), heated to reflux (65°C) and stirred for 120h. During the reaction period, acetone was regularly added, and TLC detected that the reaction was complete After that, the solution was desolvated in vacuo, and a pale yellow oily liquid was obtained by column chromatography. The yield was 75%. 1 H NMR (400MHz, CDCl 3 )δ6.88(d,J=8.1Hz,1H,Ar-H),6.73(dd,J=8.1and 1.7Hz,1H,Ar-H),6.58(d,J=1.8Hz,1H,Ar- H), 5.33–5.29 (m, 1H, CH), 3.82 (s, 1H, NH), 1.96 (d, J=1.4Hz, 3H, C=CCH) 3 ),1.27(s,6H,2CH 3 ); 13 C NMR (101MHz, CDCl 3 ) δ143.5, 128.7, 128.0, 125.0, 121.8, 121.0, 120.7, 116.1, 52.5, 30.7, 18.5, confirming that the product is 7-bromo-2,2,4-trimethyl-1,2-dihydroquinoline.
[0060] (2) Preparation of 7-brom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com