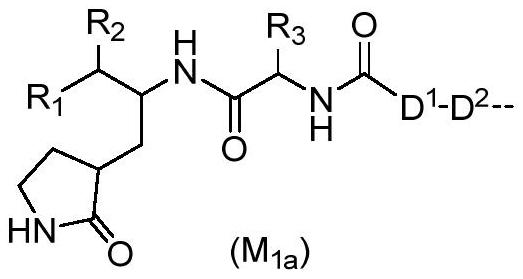
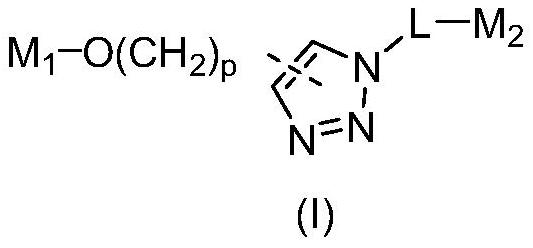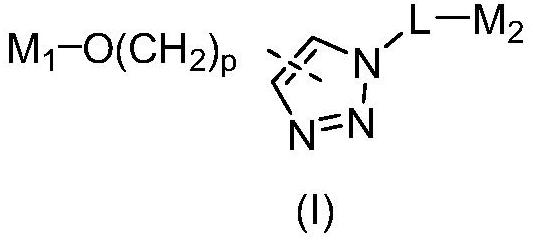PROTAC molecule targeting SARS-CoV-2 3C protease and application of PROTAC molecule
A protease inhibitor, chymotrypsin technology, applied in the field of medicine, can solve the problems such as the inability to completely inhibit the virus infection process, the inability to enter the 3C protease active protein binding domain, and the loss of the binding ability of GC376 to 3C protease.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1: Preparation of Compounds A, B and C
[0103] Compounds A1, B1 and C1 were synthesized by the following route 1, wherein compound 4 was purchased from Shanghai Shaoyuan Reagent Co., Ltd., and compound 1 and 4-O-propynyl benzyl alcohol were purchased from Aladdin Reagent Co., Ltd.
[0104]
preparation example 1-1
[0105] Preparation Example 1-1: Synthesis of Compounds A1, B1 and C1
[0106] Synthesis of intermediate 2 in step 1: Compound 1 (1.3 g, 7.60 mmol) and 4-O-propynyl benzyl alcohol (0.9 g, 5.55 mmol) were dissolved in dry acetonitrile (30 mL), and 1.5 mL of triethylamine was added, and the reaction was carried out at room temperature. , After 2 hours, the solution was concentrated, and the silica gel column was separated. Mobile phase: petroleum ether / ethyl acetate=4 / 1 to obtain 1.75 g of white solid with a yield of 95%.
[0107] Synthesis of Intermediate 3 in Step 2: Compound 2 (2.47 g, 7.73 mmol) was dissolved in 30 mL of tetrahydrofuran, 15 mL of 1M LiOH aqueous solution was added, stirred at room temperature, and after 2 hours, the system was neutralized with an acidic resin, the resin was filtered off, and concentrated , the white solid was directly used in the next reaction.
[0108] Synthesis of intermediate 5 in step 3: Compound 3 (2.1 g, 6.6 mmol) and compound 4 (1.23 ...
preparation example 1-2
[0112] Preparation Example 1-2: Synthesis of Compounds A2, B2 and C2
[0113] As shown in Scheme 1, except that 4-O-propynyl benzyl alcohol was replaced by (The compound was purchased from Jiangsu Aikang Biomedical R&D Co., Ltd.), using the same steps as the synthesis of compounds A1, B1 and C1, respectively, with the yields of 47%, 34% and 29% to obtain compound A2 as a white solid , B2 and C2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com