Nuclide-labeled inhibitory peptide as well as preparation method and application thereof
A technology for inhibiting peptides and nuclides, applied in the field of tumor prognosis, can solve problems such as adverse events, and achieve the effects of low radiation dose, good biocompatibility, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
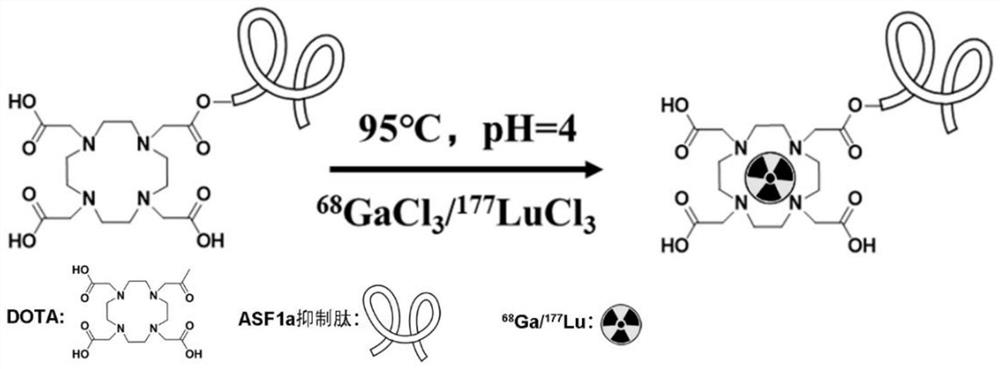
[0044] The present invention also provides a method for preparing the nuclide-labeled inhibitory peptide, comprising the following steps:
[0045] (1) Mix the DOTA-coupled ASF1a peptide with the nuclide solution to obtain a mixed solution, mix the mixed solution with sodium acetate, adjust the pH, and take a water bath to obtain a reaction solution;
[0046] (2) Pass the reaction solution obtained in step (1) through a chromatographic column to collect the product.
[0047] In the present invention, the nuclide solution in step (1) is 68 GaCl 3 solution or 177 LuCl 3 / HCl solution; preferably 68 GaCl 3 solution.
[0048] In the present invention, step (1) is described 68 GaCl 3 solution or 177 LuCl 3 The radiation dose of the / HCl solution is independently 111 to 185 MBq; preferably 121 to 175 MBq; more preferably 131 to 165 MBq; more preferably 145 MBq.
[0049] In the present invention, the 68 GaCl 3 The preparation method of the solution is: rinsing with hydroc...
Embodiment 1
[0069] Synthesis of ASF1aPeptide, AP1
[0070] (1) Estimate the feeding amount of each amino acid according to the weight and molecular weight of the target polypeptide, and each amino acid and DOTA use protective raw materials.
[0071] (2) put 2-Cl(Trt)-Cl resin into 150mL reactor, and add 80mL DCM to soak for 2 hours;
[0072] (3) The resin was washed with DMF, and then drained. This was repeated 4 times to drain the resin.
[0073] (4) Weigh Fmoc-Ala-OH (the first amino acid at the C-terminal, CAS No. 154445-77-9) + 80 mL of DCM and DIEA into the reactor, and then place the reactor in a shaker at 30°C reaction for 2 hours;
[0074] (5) Add 0.5 mL of DIEA and 0.5 mL of methanol with a pipette, react for 20 min, and block unreacted groups on the resin.
[0075] (6) add an appropriate volume ratio of 20% piperidine solution (piperidine / DMF=1:4) into the reactor, which is 3 times the volume of the resin, react for 20min, remove the Fmoc protecting group, and use DMF washed...
Embodiment 2
[0085] A nuclide-labeled inhibitory peptide, the nuclide-labeled inhibitory peptide is converted to 68 Ga-labeled ASF1a peptide; the amino acid sequence of the ASF1a peptide is YGRKKRRQRRRCASTEEKWARLARRIAGAGGVTLDGFGGCA;
[0086] The preparation method of the nuclide-labeled inhibitory peptide, the steps are as follows:
[0087] (1) Rinse with 4 mL of 0.05M hydrochloric acid 68 Ge- 68 Ga generator (flow rate is 0.8mL / min), collect the 2~3mL intermediate product 68 GaCl 3 ;
[0088] (2) 15 μL of DOTA-conjugated ASF1a peptide (at a concentration of 1 mg / mL) was mixed with 1 mL 68 GaCl 3 The solution (radiation dose of 111MBq) was mixed to obtain a mixed solution, the mixed solution was mixed with 250 μL of sodium acetate (concentration of 0.23M), the pH was adjusted to 3.8, and the reaction solution was obtained in a metal bath at 93°C for 8 minutes;
[0089] (3) 5 mL of 70% ethanol was used to activate the C18 cartridge dropwise, rinsed with 5 mL of normal saline, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com