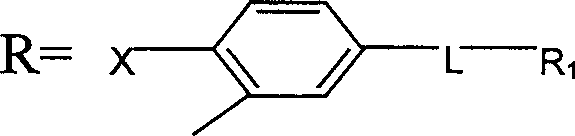
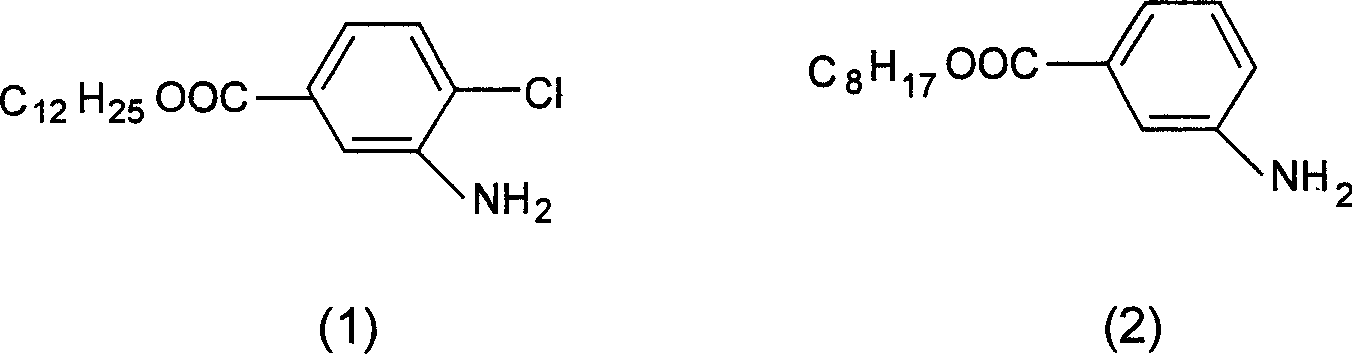Preparing process of propane diamide compound
A technology of malonamide and compound, applied in the field of preparation of malonamide compounds, can solve the problems of high reaction temperature, long reaction time, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of compound (1)'
[0026]
[0027] Add 170g of 4-chloro-3-amino-dodecyl benzoate, 26g of malonic acid and 1000ml of benzene into a 2000ml three-neck flask, heat to 60°C, add 37.5ml of phosphorus trichloride dropwise within half an hour, and continue The reaction was carried out at this temperature for 1.5 hours, and the benzene solvent was distilled off to obtain 183 g of a white solid with a yield of 98% and a melting point of 94-96°C.
[0028] In the case of other reaction conditions being the same, the temperature was controlled at 62°C and 65°C respectively, and the above compounds were synthesized, and the obtained results were the same.
[0029] C
Embodiment 2
[0031] Synthesis of compound (2)'
[0032] Add 24.9g of n-octyl m-amino-benzoate, 5.2g of malonic acid and 150ml of chlorobenzene into a 500ml three-neck flask, heat to 62°C, add 5.2ml of phosphorus trichloride dropwise within 1 hour, and continue the reaction for 2.0 After 1 hour, the chlorobenzene solvent was distilled off to obtain 26.9 g of a white solid with a yield of 95% and a melting point of 72-75°C.
[0033] In the case of other reaction conditions being the same, the temperature was controlled at 60°C and 65°C respectively, and the above compounds were synthesized, and the obtained results were the same.
[0034] C
Embodiment 3
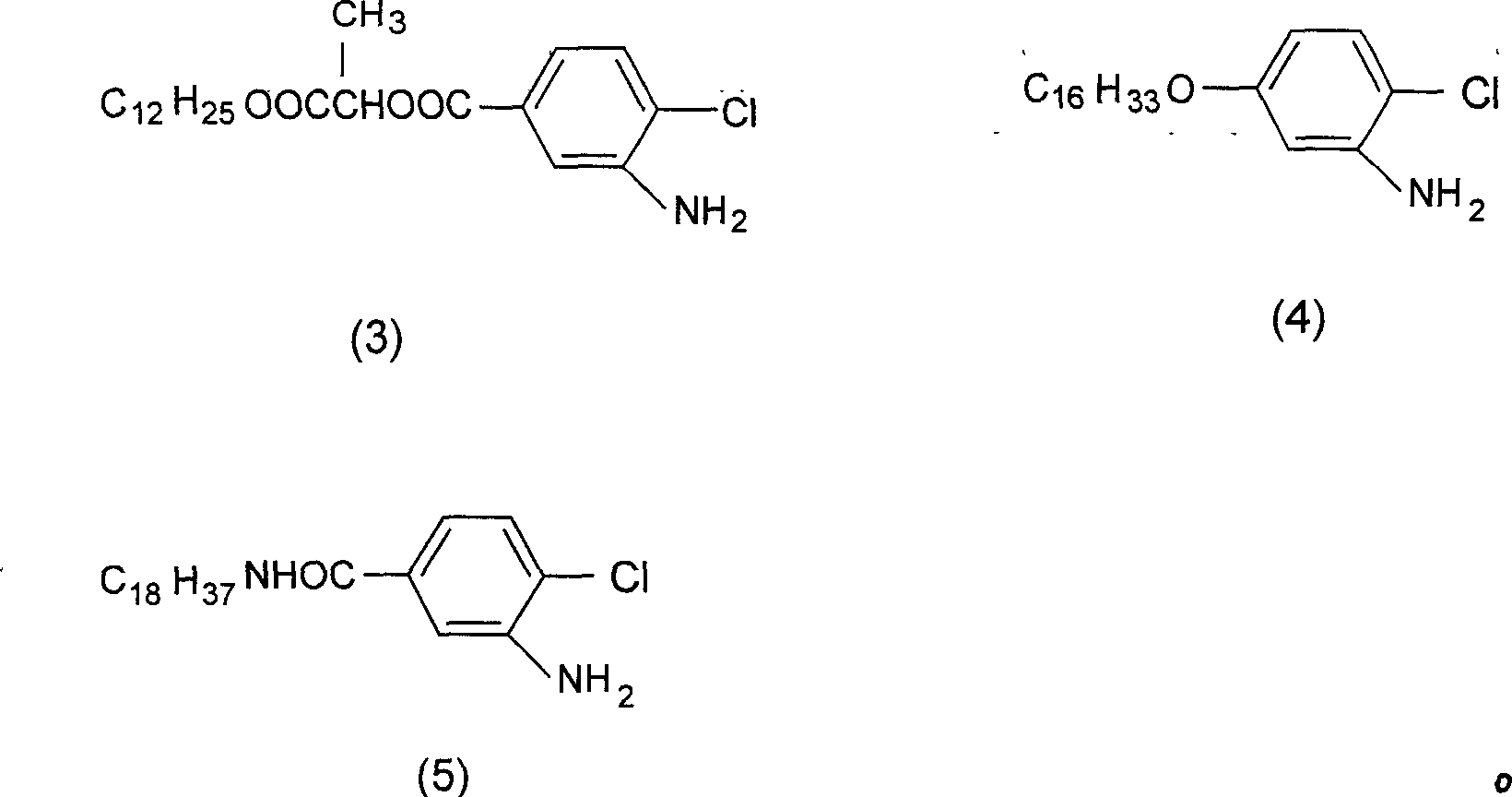
[0036] Synthesis of compound (3)'
[0037] Add 41.1g of 4-chloro-3-amino-benzoic acid (α-dodecyl methyl acetate), 5.2g of malonic acid and 180ml of benzene into a 500ml three-necked flask, heat to 65°C, and add 5.2ml dropwise within 1 hour After the addition of phosphorus trichloride, the reaction was continued for 1.5 hours, and the benzene solvent was evaporated to obtain 40 g of a white solid with a yield of 90% and a melting point of 66-67°C.
[0038] In the case of other reaction conditions being the same, the temperature was controlled at 60°C and 63°C respectively, and the above compounds were synthesized, and the results obtained were the same.
[0039] C
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com