Method for low-temperature preparation of pure phase oxide material
An oxide and pure-phase technology, which is applied in the field of low-temperature preparation of pure-phase oxide materials, can solve the problems of lowering the synthesis temperature, limiting practical applications, and short reaction time, so as to reduce the synthesis reaction temperature, overcome uneven mixing and liquid phase The process is complex and the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
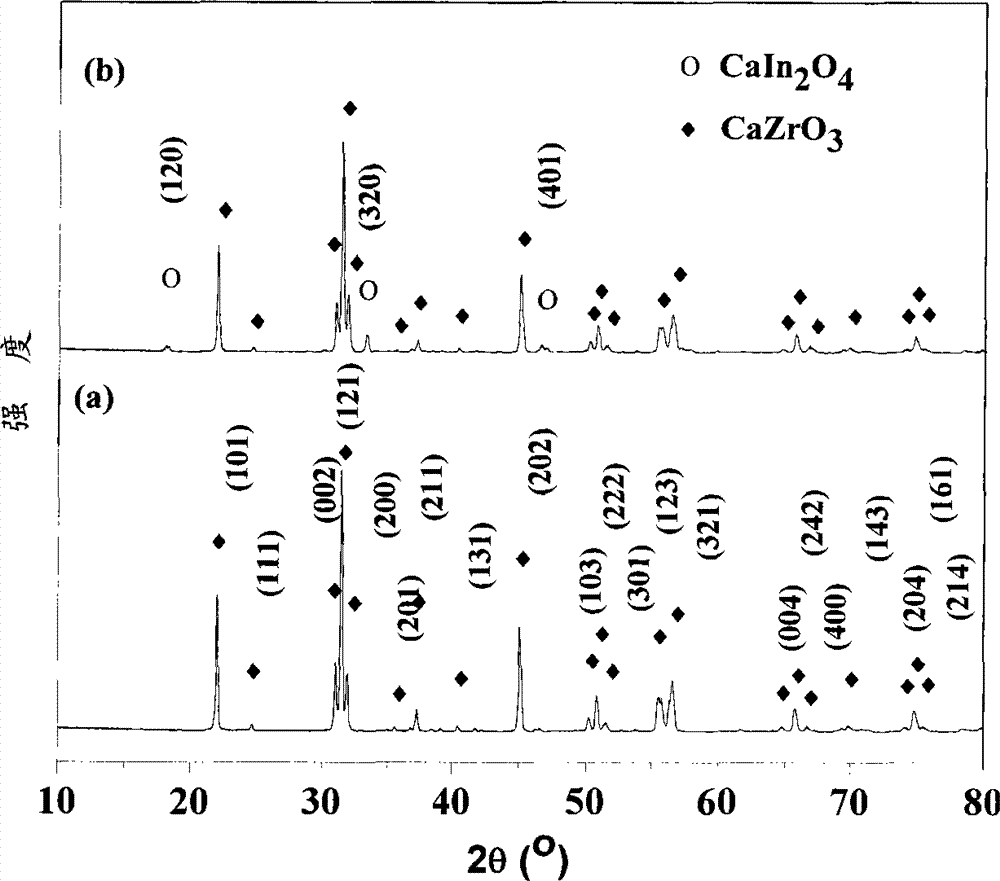
[0027] 0.045 moles (19.32 grams) of Zr(NO 3 ) 4 ·5H 2 O and 0.005 mol (1.91 g) In(NO3 ) 3 4.5H 2 O was dissolved in 100 ml of distilled water and stirred well, then slowly added 5.00 g of CaCO 3 , and stir until all the calcium carbonate is dissolved. Add 5ml of PEG200 (polyethylene glycol) to the above solution, and continue stirring until the solution turns into a gel. React the gel at 850°C for 2 hours to obtain a uniformly dispersed high-purity CaZr of about 60 nanometers 0.90 In 0.10 o 2.95 Powder. More than 99% dense ceramics can be obtained by sintering at 1350°C for 6 hours. Mechanical tests show that the bending resistance of ceramics reaches 283Mpa; the results of proton conductivity test show that the proton conductivity of grain boundaries in hydrogen-containing and water-containing atmospheres is 2.64×10 at 800°C. -3 S cm -1 , the bulk proton conductivity is 8.72×10 -4 S cm -1 The conductance of the grain boundary is greatly improved, and the conductan...
Embodiment 2
[0029] 0.05 moles (21.47 grams) of Zr(NO 3 ) 4 ·5H 2 O and 0.05 mol (19.1 g) In(NO 3 ) 3 4.5H 2 O was dissolved in 200 ml of distilled water and stirred well, then slowly added 22.53 g of BaC 2 o 4 , and stir until the barium oxalate is completely dissolved. Add 20ml of PEG400 (polyethylene glycol) to the above solution, and continue stirring until the solution turns into a gel. The gel was reacted at 700°C for 10 hours to obtain a uniformly dispersed 60nm white powder BaZr 0.50 In 0.50 o 2.75 . Ceramics with a density greater than 98% can be obtained by sintering at 1400°C for 5 hours. The proton conductivity test results show that the ceramics obtained by pressing and sintering the powder obtained by this method have good proton conductivity. At 800°C, the proton conductivity can reach 10 -3 order of magnitude.
Embodiment 3
[0031] 0.09 mol (39.08 g) Ce(NO 3 ) 3 ·6H 2 O and 0.01 mol (4.34 g) La(NO 3 ) 3 ·6H 2 O was dissolved in 200 ml of distilled water and stirred evenly, then slowly added 17.13 g of Ba(OH) 2 , and stir until the barium hydroxide is completely dissolved. Add 10ml of PVP (polyvinylpyrrolidone) to the above solution, and continue stirring until the solution turns into a gel. The gel was reacted at 850°C for 6 hours to obtain uniformly dispersed 80nm BaCe 0.90 ln 0.10 o 2.95 Powder. Under wet hydrogen, the conductivity can reach 10 at 1000°C -1 Magnitude.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flexural strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com