Benzofurazan compounds which enhance AMPA receptor activity
The technology of a compound, benzofurazan, is applied in the field of prevention and treatment of cerebral insufficiency, which can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] III. Preparation of Compounds of the Invention
[0043] The compounds of the present invention can be synthesized by various methods using conventional synthetic chemistry techniques. The preparation method of the compound of the present invention includes as follows.
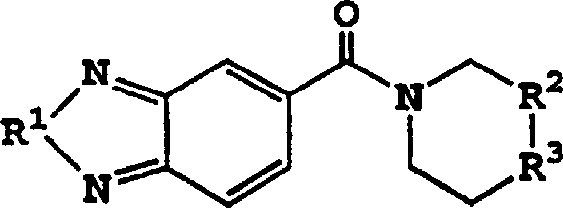
[0044] where R 4 and R 8 Compounds of the invention which do not form a linking moiety may be obtained according to figure 1 Preparation as shown: the carboxyl group of suitably substituted benzoic acid or nicotinic acid, pyrazinic acid, pyridizine (pyridizine) carboxylic acid or pyrimidine carboxylic acid is treated with carbonyldiimidazole or other reactive groups such as thionyl chloride in anhydrous solvent such as Activate in dichloromethane, chloroform, tetrahydrofuran or ethyl acetate. The cyclic amine is then reacted with the activated carboxyl group. According to the above preferred structures, the cyclic amine preferably comprises an optionally substituted piperidine derivative. Rings may...
Embodiment 1
[0099] Example 1: 1-(Benzo-2.1.3-thiadiazol-5-ylcarbonyl)piperidine (1)
[0100] Trimethylaluminum (2M in toluene; 3.0ml, 6.0mmol) was diluted with 30ml of dichloromethane and piperidine (0.55g, 6.5mmol) and benzo-2,1,3-thiadiazole- 5-Methyl carboxylate (1.16 g, 6.00 mmol). The reaction was stirred at room temperature for 2 hours, then concentrated to half its original volume by rotary evaporation. Dry toluene (25ml) was added and the reaction was heated at 80°C for 1 hour. Additional piperidine (ca. 0.2 g) was added and the temperature was raised to 100° C. for 1 hour. The solution was cooled to room temperature and stirred overnight, then quenched with 10% citric acid and hydrochloric acid. The solution was diluted with ethyl acetate, washed successively with 10% citric acid, saturated sodium hydrogenphosphate solution and saturated sodium chloride solution, and then dried over anhydrous sodium sulfate. The solution was concentrated on silica gel and the product was elut...
Embodiment 2
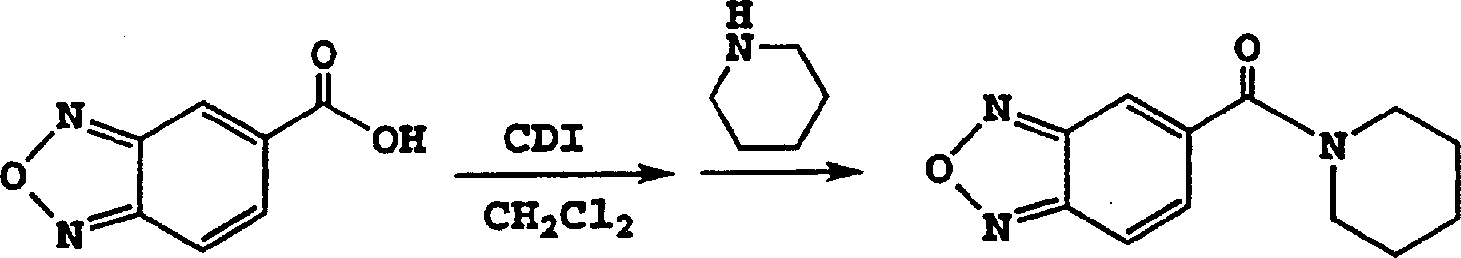
[0101] Example 2: 1-(benzofurazan-5-ylcarbonyl)piperidine (2)
[0102] Method A:
[0103] Benzofurazan-5-carboxylic acid (2.0 g, 12.2 mmol) was suspended in 10 ml of dichloromethane. Carbonyldiimidazole (2.0 g, 12.3 mmol) was added and the suspension began to dissolve with gas evolution. The resulting yellow solution was stirred at room temperature for 40 minutes, then piperidine (1.2 g, 14.1 mmol) was added. The solution was stirred overnight, then concentrated onto silica gel. The product was eluted with hexane / ethyl acetate (2:1) and purified by distillation in a kugelrohr at 155-170° C. and 0.5 mmHg to give 1-(benzofurazan-5-ylcarbonyl)piperidine, 2 (2.78 g, 99%), the product was initially a light yellow oil which crystallized on cooling.
[0104] IR: 2938, 2857, 1630, 1519, 1439, 1266, 1223, 996, 881, 816, and 740cm -1 . 1 H NMR (500MHz): δ7.90 (1H, d, J = 9.7Hz); 7.84 (1H, s); 7.44 (1H, dd, J = 9.4 and 1.4Hz); 3.74 (2H, br s); 3.39 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com