Urea compounds having muscarinic receptor antagonist activity
A compound and nitrogen atom technology, applied in organic active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as blurred vision, application restrictions, dry mouth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0304]Preparation of compounds of formula (I)
[0305] In general, compounds of formula (I) can be prepared as illustrated and described in Reaction Scheme A.
[0306] Reaction Scheme A
[0307]
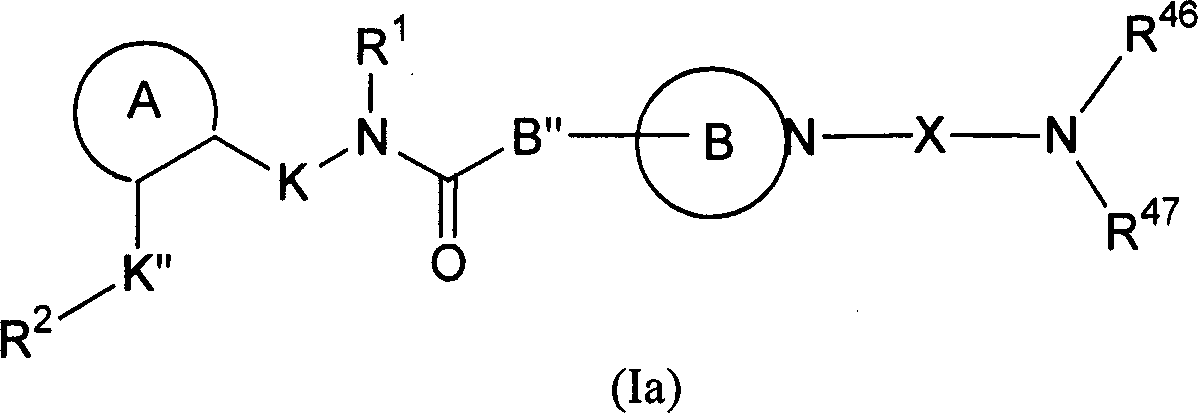
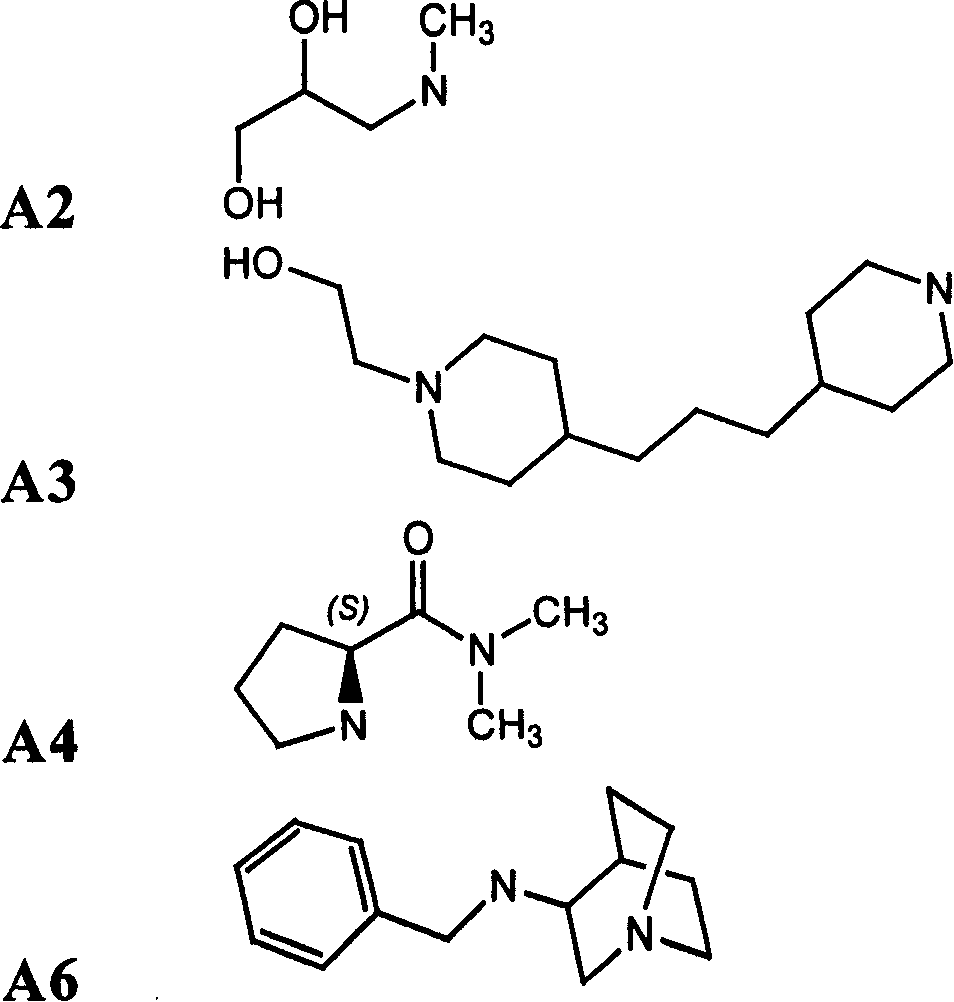
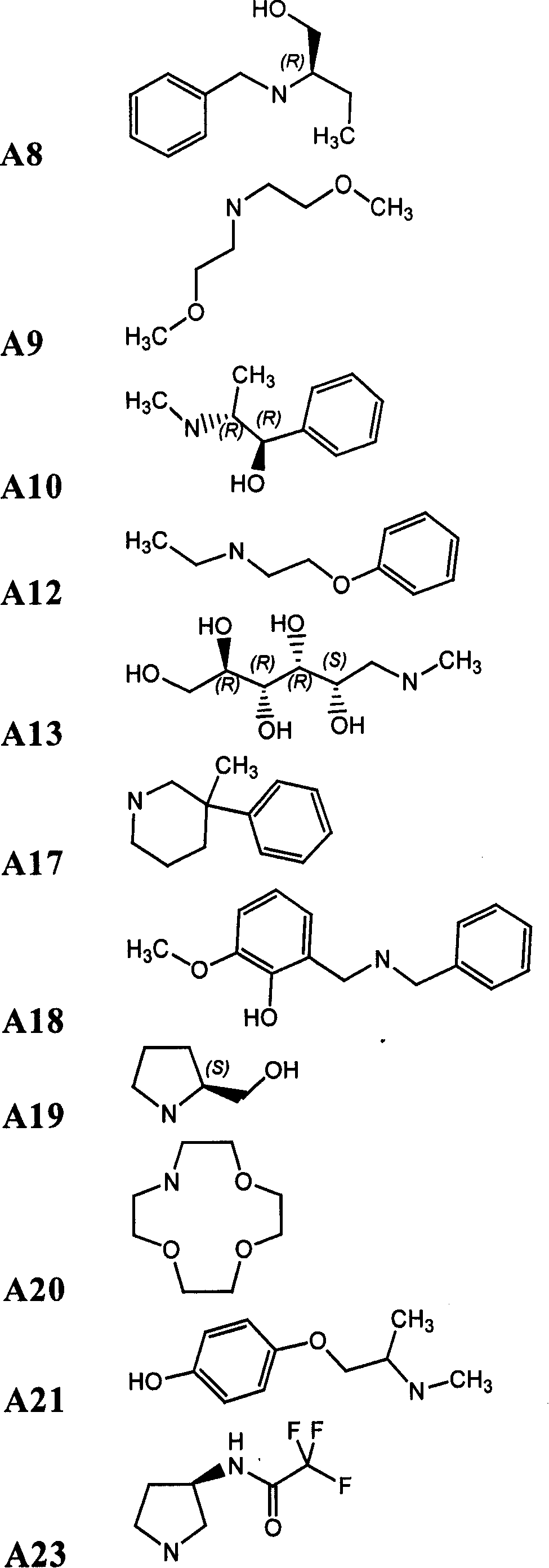
[0308] Compounds of formula (I) are prepared by the following method: 1 equivalent formula 1 Compounds and Formulas 2 Compounds are covalently linked to form intermediates of formula (II), wherein X is a linker as defined herein, FG 1 is the functional base, FG 2 is with FG 1 Complementary functional group, PG is a protecting group, FG 2 PG is a protected functional group. The functional group on the linker is deprotected, and the resulting compound 3 and 1 equivalent compound 4 The reaction yields the compound of formula (I). compound 1 and 4 with compound 2 and 3 The reaction conditions used for ligation depend on the compound 1 , 2 , 3 and 4 on the nature of the functional group, which in turn depends on the type of bond required. Functional groups and...
Embodiment 1
[0368] Intermediate compounds of formula IB are prepared as described below.
[0369]
[0370] Biphenyl-2-isocyanate (50 g, 256 mmol) was dissolved in 400 ml of anhydrous acetonitrile in a 2 L round bottom flask at room temperature. After cooling to 0°C with an ice bath, a solution of 4-amino-N-benzylpiperidine (48.8 g, 256 rnol) dissolved in 400 mL of anhydrous acetonitrile was added over a period of 5 minutes. The formation of a precipitate was immediately observed. After 15 minutes, an additional 600 mL of dry acetonitrile was added and the viscous solution was stirred at 35°C for 12 hours. The solid was filtered off, washed with cold acetonitrile and dried in vacuo to give a colorless solid (100 g, 98%). pass the substance through 1 H-NMR, 13 C-NMR and MS were used for identification.
[0371] Compound IA (20 g, 52 mmol) was dissolved in 800 mL of a 3:1 mixture of anhydrous methanol / anhydrous DMF. Aqueous HCl (0.75 mL of a 37% concentrated solution, 7.6 mmol) was ...
preparation Embodiment
[0392] Example 1
[0393] Hard gelatin capsules are prepared containing the following ingredients:
[0394] quantity
[0395] Ingredients (mg / capsule)
[0396] Active ingredient 30.0
[0397] Starch 305.0
[0399] The above ingredients were mixed and filled into hard gelatin capsules in an amount of 340 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com