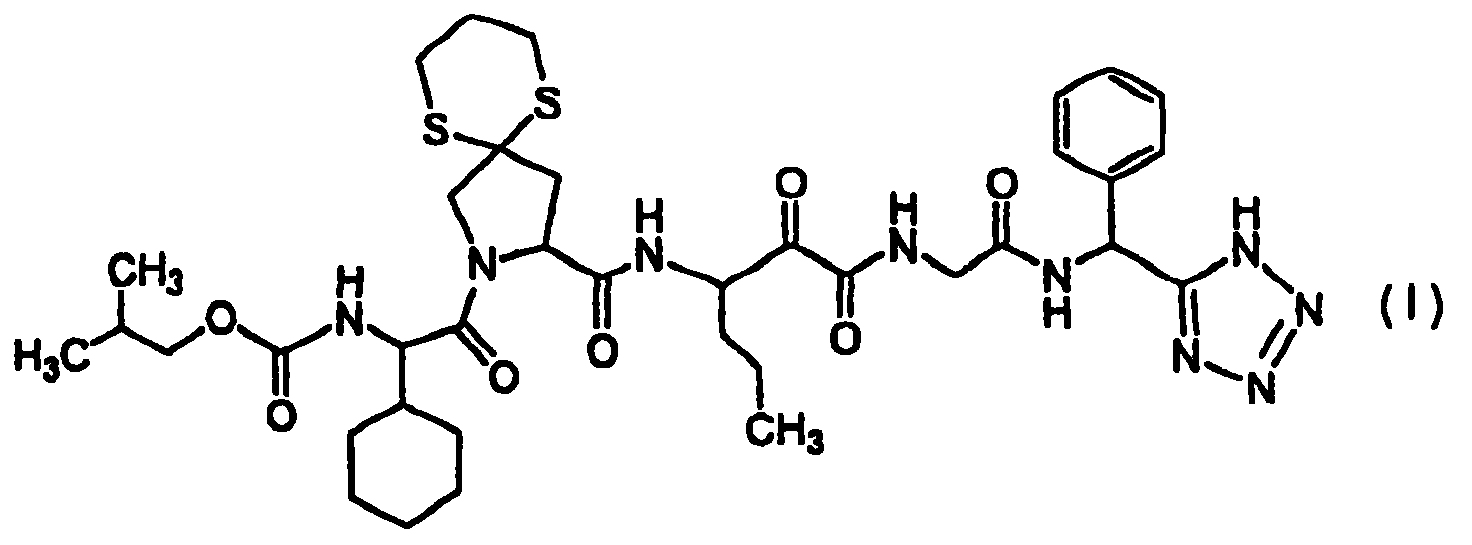
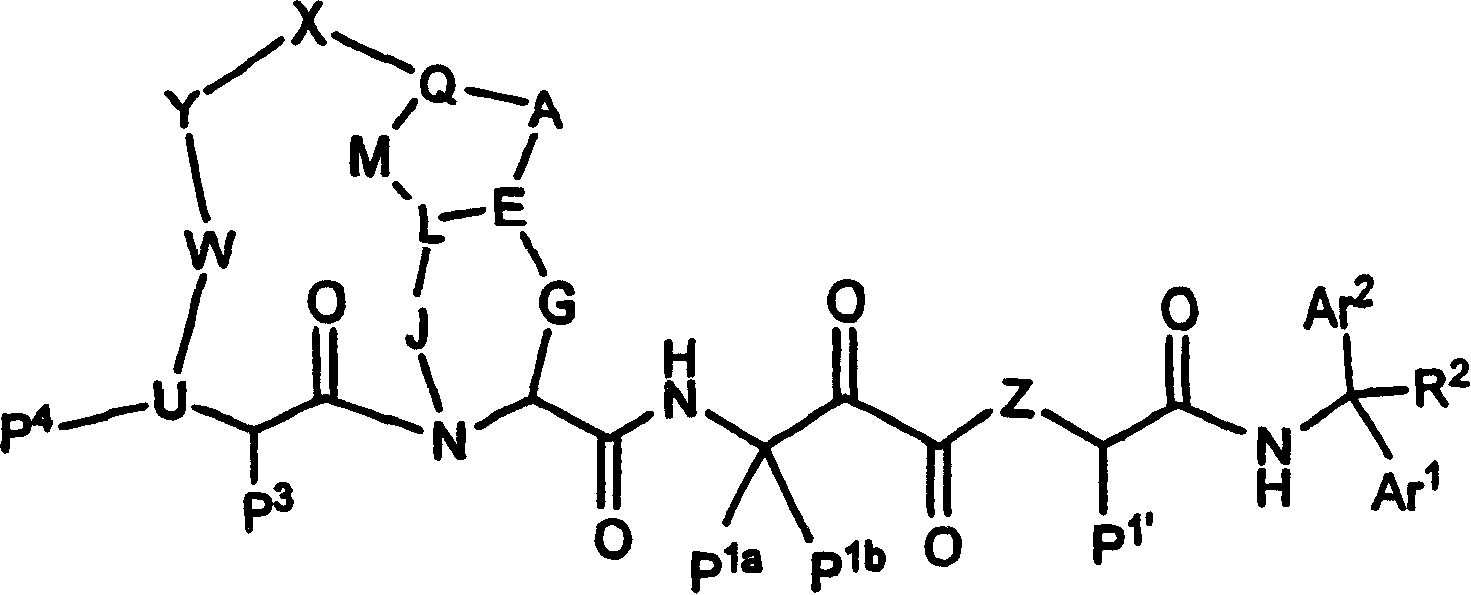
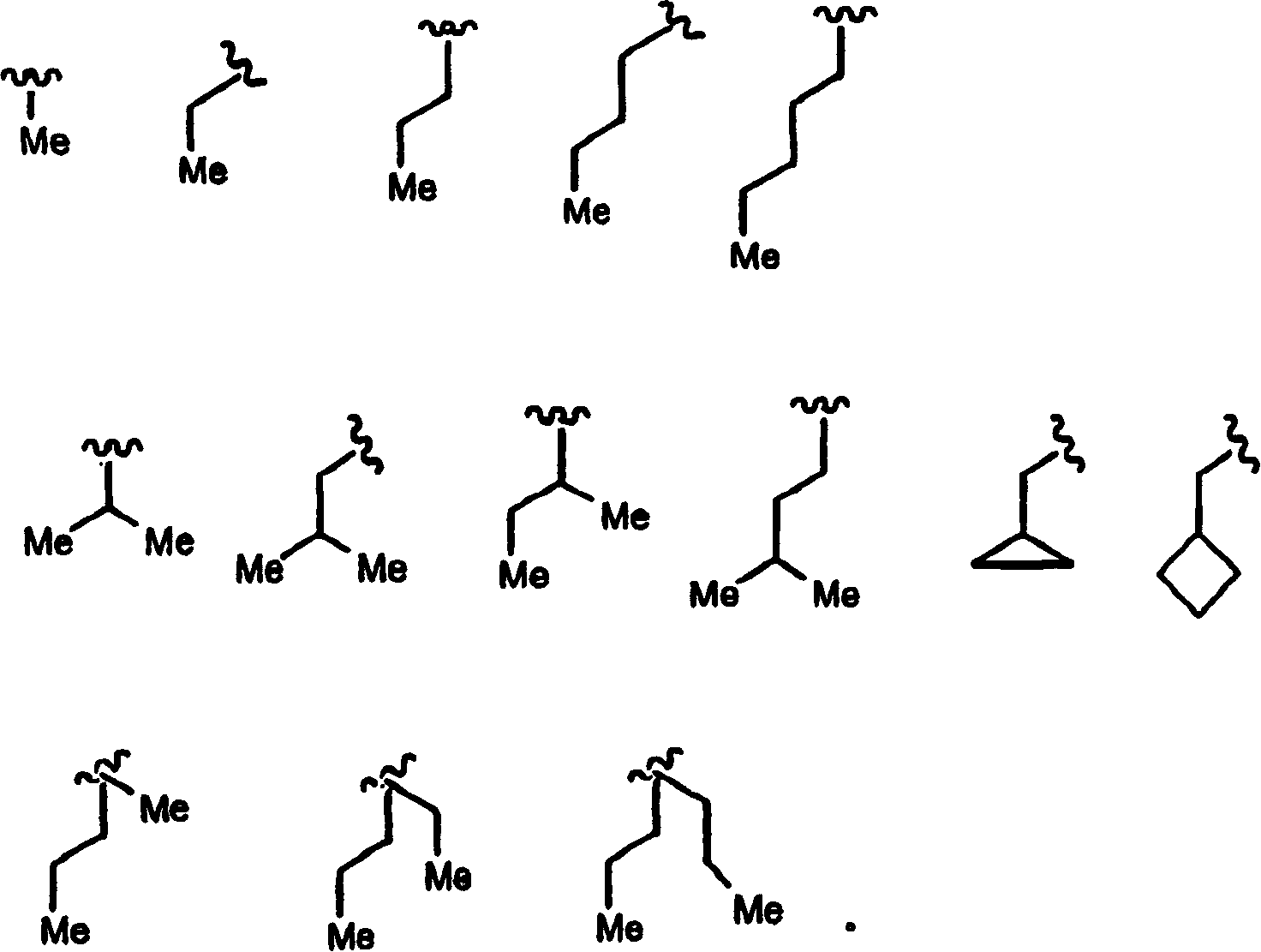
Diaryl peptides as ns3-serine protease inhibitors of hepatitis c virus
An alkyl and group technology is applied in the field of diaryl peptides as hepatitis C virus NS3-serine protease inhibitors, which can solve the problem of lack of vaccines and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0290] If necessary, some intermediates that are not commercially available can be synthesized according to the following steps:
[0291]
[0292] II. Methanesulfonate:
[0293] A mixture of triphenylphosphine (8.7 g), toluene (200 ml) and methanesulfonic acid (2.07 ml) was stirred at 15° C. while slowly adding diethyl azidedicarboxylate (7.18 g) to maintain the temperature below 35°C. The mixture was cooled to 20° C., N-Boc amino acid (7.4 g, BachemBiosciences, Inc.) and Et 3 N (1.45 mL), and the mixture was stirred at 70°C for 5 hours. The mixture was cooled to 5°C, the organic supernatant was decanted and the solvent was removed in vacuo. Residue and Et 2 O (200 mL) was stirred together until a precipitate settled out, the mixture was filtered and the ether solution was chromatographed on silica gel (5:95 to 20:80 EtOAc-Et 2 O) The product (9.3 g) was obtained and used for the next step.
[0294] III. Azides
Embodiment 1
[0303] Step A
[0304]
[0305] To compound (4.01) (12 g) prepared according to S.L.Harbeson et al., J.Med.Chem.37(18), 2918-2929 (1994) at -20°C in CH 2 Cl 2 To a stirred solution in (150 mL) was added HOOBt (7.5 g), N-methylmorpholine (6.0 mL) and EDCl (10 g). The reaction mixture was stirred for 10 min, followed by the addition of HCl·H 2 N-Gly-OMe (6.8 grams). The resulting solution was stirred at -20°C for 2 hours, then at 8°C overnight. The solution was concentrated to dryness and diluted with EtOAc (150 mL). EtOAc followed by saturated NaHCO 3 、H 2 O, 5%H 3 PO 4 Wash the solution twice with brine and wash with Na 2 SO 4 Drying, filtration and concentration to dryness afforded the product. C 14 h 26 N 2 o 6 (318.37)LRMS m / z MH + = 319.3.
[0306] Example 1
[0307] Step B
[0308]
[0309] A mixture of the product from Step A above (5.7 g), dichloromethane (200 mL), dimethylsulfoxide (12 mL) and 2,2-dichloroacetic acid (3.2 mL) was stirred at 5°C...
Embodiment 2
[0323] Step A
[0324]
[0325] N-Boc-phenylglycine N-hydroxysuccinimide ester (1.66 g, BachemBiosciences, Inc.) in dichloromethane (CH 2 Cl 2 , 20 ml) at 5°C with 0.5M NH 3 / dioxane (Aldrich Chemical Co.) (18.5 mL) solution followed by warming to room temperature and stirring for 4 hours. Suction filter the mixture and add the filtrate to 5% KH 2 PO 4 aqueous solution (150 mL), followed by extraction with ethyl acetate (EtOAc, 200 mL). Extract with 5% KH 2 PO 4 The aqueous solution (twice) was then washed with saturated brine. Extraction with anhydrous MgSO 4 Drying, filtering the mixture and concentrating the filtrate in vacuo left the crude title compound (1.15 g) which was used directly in the next step.
[0326] Example 2
[0327] Step B
[0328]
[0329] A solution of the product of the preceding step (1.15 g) in pyridine (10 ml) was treated with POCl at 5 °C 3 (0.6 mL) followed by warming to room temperature and stirring for 3 hours. The mixture was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com