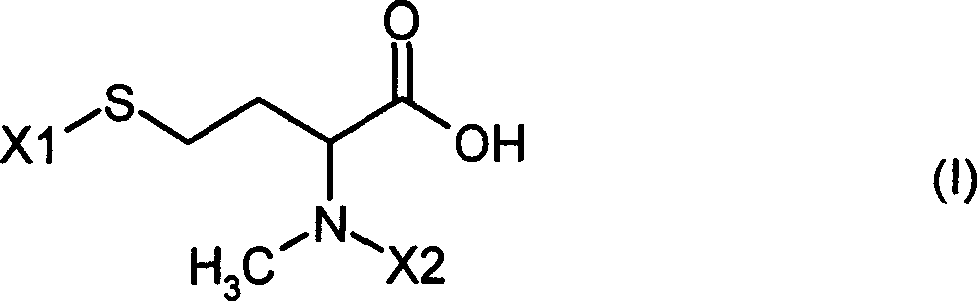
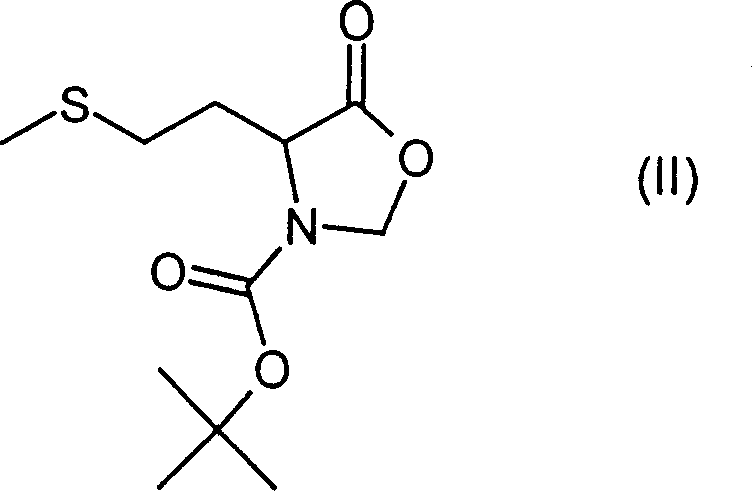
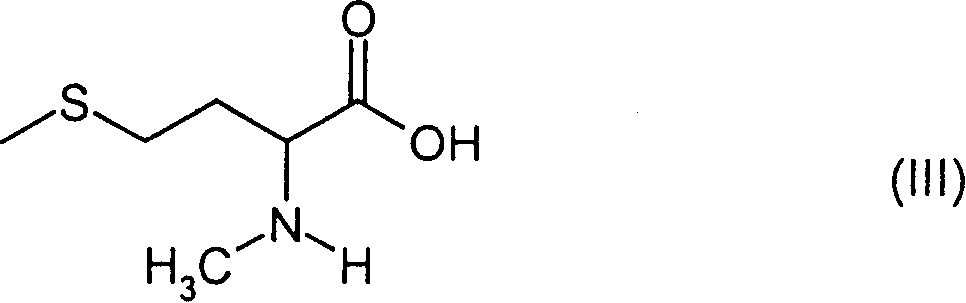
N-methyl-homocysteines and their use as well as process for their production
A technology of methyl and ylmethyloxycarbonyl, which is applied to N-methyl-homocysteine and its application and preparation fields, can solve the problem that the yield is only 22% and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] a) N-(tert-oxycarbonyl)- A mixture of L-methionine (0.4mol), 100g of paraformaldehyde (3.3mol), 200g of anhydrous magnesium sulfate (1.7mol) and 4g of p-toluenesulfonic acid (0.02mol) in 1L of toluene was heated for 3 hours to 90 ℃. After cooling to 20°C, 800 ml of a saturated sodium bicarbonate solution was added thereto while cooling with ice. It was filtered and the residue was washed with 400 ml of ethyl acetate. The organic phase is extracted with 300 ml of water, dried over sodium sulfate and evaporated to dryness in vacuo. The crude product was dissolved in 150 ml of hexane / ethyl acetate 1:3, then filtered on silica gel and washed again with 500 ml of hexane / ethyl acetate 1:3. Evaporation to dryness in vacuo gave 85.3 g (0.33 mmol, 83% of theory) of a yellow oil.
[0028] Calculated: C 50.56 H 7.33 N 5.36 S 12.27
[0029] Found: C 50.38 H 7.24 N 5.43 S 12.10
[0030] IR: 2980, 2920, 1800, 1715, 1390, 1170, 1050
[0031] NMR (CDCl 3 ): 1.5(9H), 2.1(3H), 2....
Embodiment 2
[0056] Using N-(tertoxycarbonyl)-D-methionine in place of N-(tertoxycarbonyl)-L-methionine as described above, the enantiomeric compound of the title compound of Example 1d can be obtained similarly.
[0057] a) tert-butyl-(R)-4-[2-(methylsulfanyl)ethyl)]-5-oxooxazolidinone-3-carboxylate
[0058] Calculated: C 50.56 H 7.33 N 5.36 S 12.27
[0059] Found: C 50.30 H 7.51 N 5.50 S 12.41
[0060] IR: 2980, 2920, 1800, 1715, 1390, 1175, 1050
[0061] NMR (CDCl 3 ): 1.5(9H), 2.08(3H), 2.15-2.35(2H), 2.5-2.68(2H), 4.33(1H), 5.21(1H), 5.48(1H)
[0062] MS(EI): m / z 261, 205, 188, 116, 100, 57
[0063] b) N-methyl-D-methionine
[0064] Calculated: C 44.15 H 8.03 N 8.58 S 19.64
[0065] Found: C 43.98 H 7.81 N 8.70 S 19.82
[0066] IR: 3430, 3010, 2920, 2830, 2420, 1630, 1580, 1395
[0067] NMR (D 2 O): 2.13(3H), 2.12-2.22(2H), 2.55-2.68(2H), 2.74(3H), 3.68-3.73(1H)
[0068] MS(EI): m / z 163, 145, 118, 88, 70, 61
[0069] CD (water): 200nm, Δε 1.87
[0070] c) N-methyl-S-trityl...
Embodiment 3
[0083] N-Benzyloxycarbonyl-N-methyl-S-trityl-L-homocysteine
[0084] 16.6 g (0.042 mol) of N-methyl-S-trityl-L-homocysteine (the title compound of Example 1c) were suspended in 90 ml of water and 100 ml of THF, then 12.58 g of (0.12 mol) of sodium carbonate. At 10° C., a solution of 10.97 g (0.044 mol) of N-benzyloxycarbonyloxysuccinimide in 50 ml THF is added dropwise and stirred at 10° C. for a further 3 hours. It is then mixed with 80 ml of water and 130 ml of ethyl acetate, stirred at room temperature for 10 minutes, and then brought to pH 4 with citric acid. The organic phase was washed twice with 100 ml of water and once with 100 ml of common salt solution. The aqueous phase was then extracted once more with 100 ml of ethyl acetate. The combined organic phases are evaporated to dryness in a vacuum; the residue is chromatographed on silica gel (mobile solvent: dichloromethane / acetone 6:4). This gave 22.08 g (87% of theory) of the product as a solid colorless foam. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com