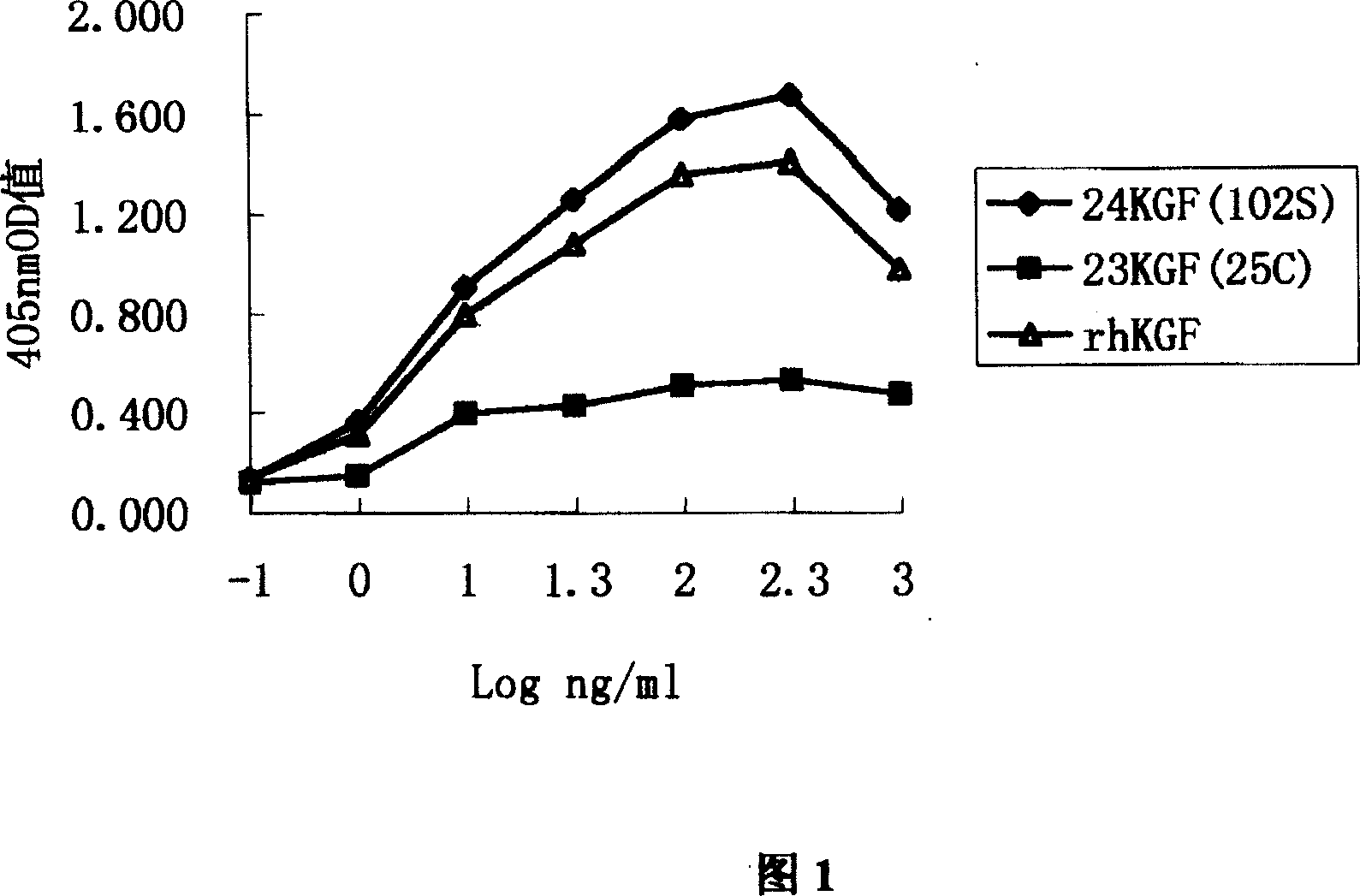
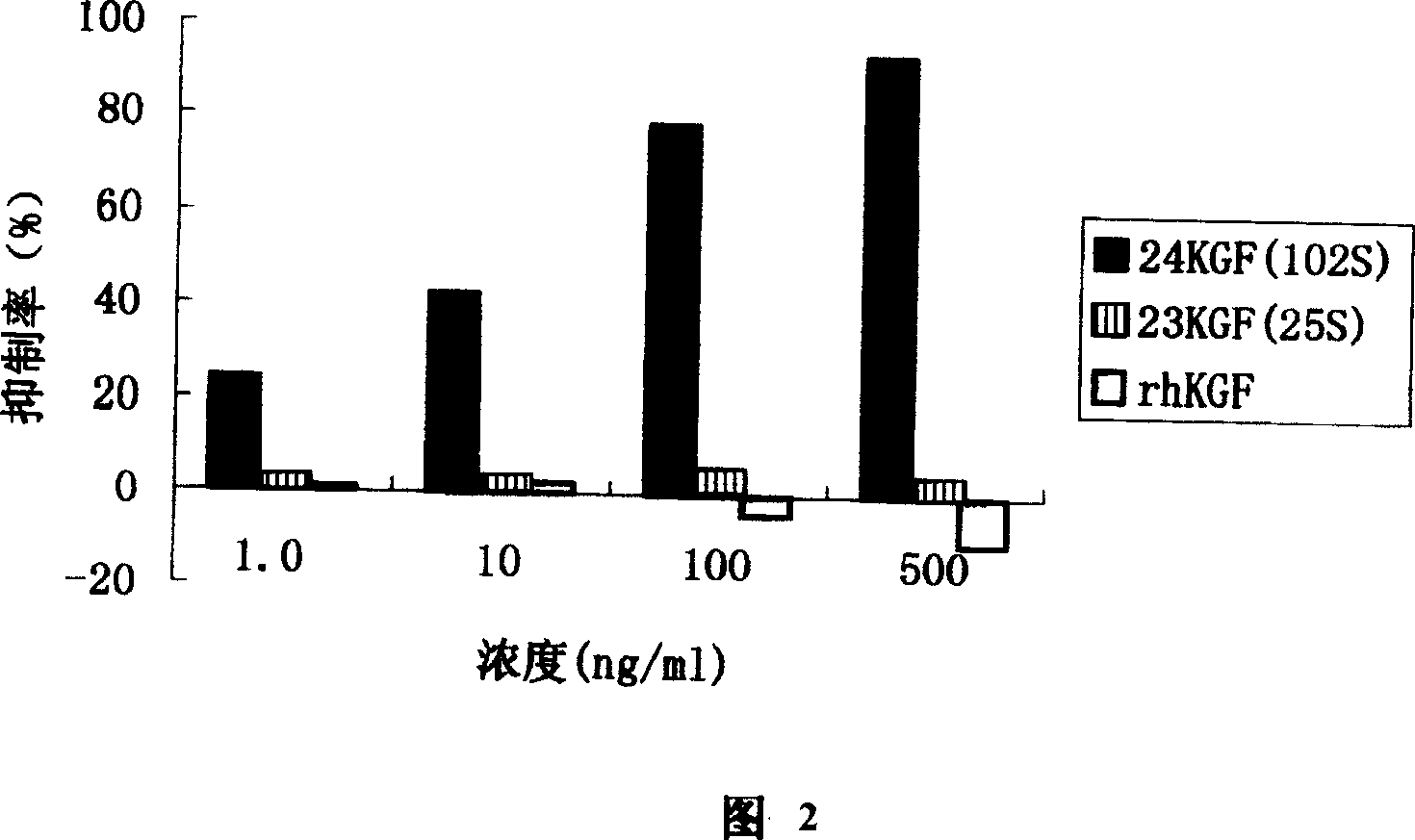
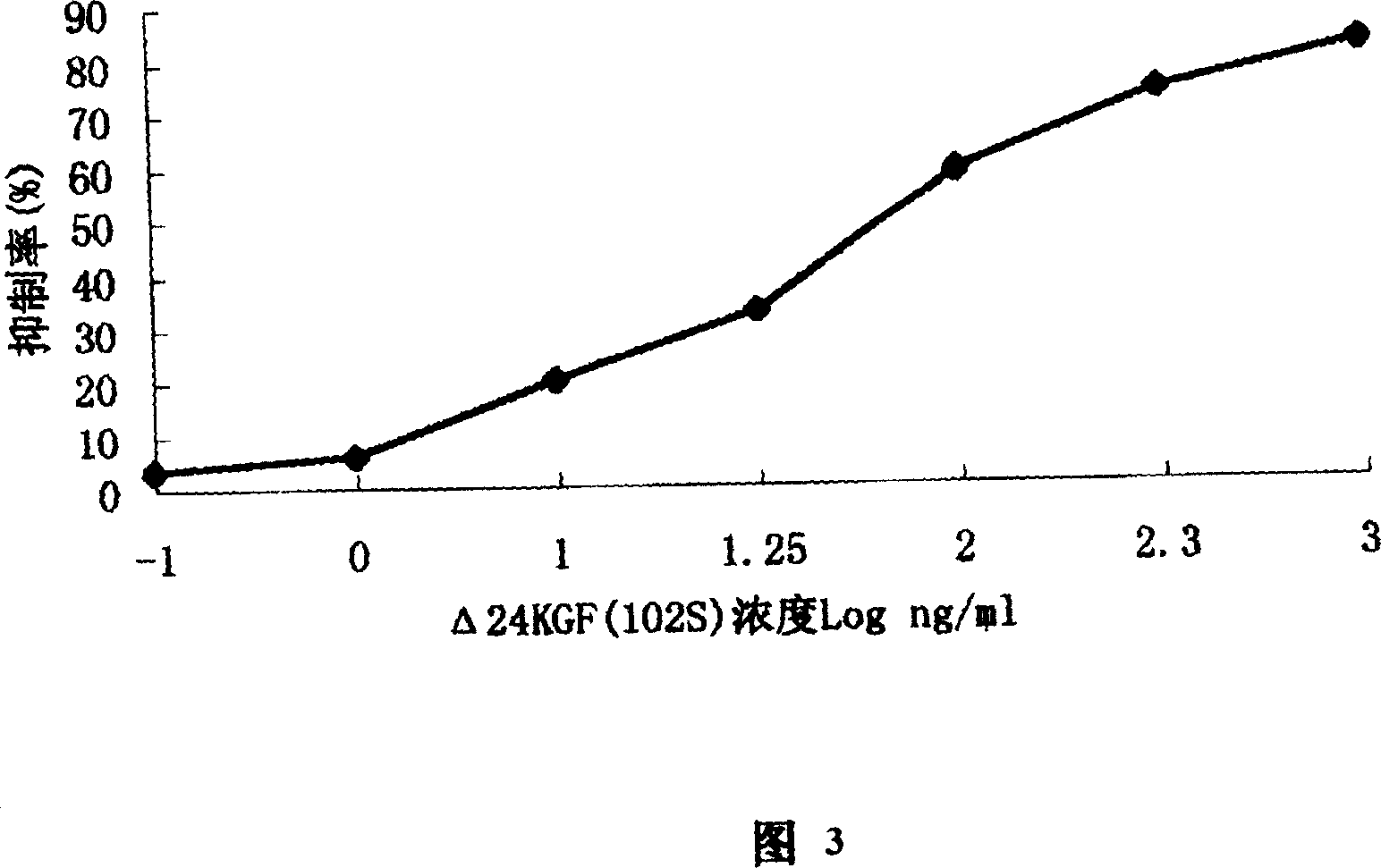
Novel human keratinocyte growth factor mutant
A keratinocyte and growth factor technology, applied in growth factors/inducing factors, animal/human proteins, peptides/protein components, etc., can solve problems such as reducing the biological activity and stability of expressed proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Example 1: Human keratinocyte growth factor (KGF) whole gene artificial synthesis scheme
[0017] According to the human KGF amino acid sequence (composed of 163 amino acids) reported in GenBank and literature, the full gene sequence of Δ23KGF was artificially synthesized by recursive PCR method, and the Nde I and EcoR I sites contained in it were changed. Escherichia coli or yeast preferred codons were selected for artificial synthesis in order to increase the level of recombinant expression.
[0018] Seq1: amino acid sequence of human KGF
[0019] cndmt peqma tnvnc ssper htrsy dymeg gdirv rrlfc rtqwy lridk rgkvk gtqem
[0020] 30 60
[0021] knnyn imeir tvavg ivaik gvese fylam nkegk lyakk ecned cnfke lilen hynty
[0022] 90 120
[0023] asakw thngg emfva lnqkg ipvrg kktkk eqkta hflpm ait
[0024] 150
[0025] Seq2: cDNA sequence of artificially synthesized human Δ23...
example 2
[0051] Example 2: Construction of the recombinant plasmid of the Δ24KGF (102S) mutant
[0052] Δ24KGF (102S) is a novel KGF mutant in which the cysteine at position 102 is mutated to serine on the basis of the deletion of 24 amino acids at the N-terminal of human KGF. Taking the artificially synthesized human KGFcDNA fragment in Example 1 as a template, two pairs of primers were synthesized for PCR-guided directed point mutation and deletion.
[0053] Seq3: (forward) 5'-CATATGTACGACTACATGGAAGGTGGTGAC-3' (for N-terminal deletion)
[0054] Seq4: (reverse) 5'-TTCGTTGGATTCTTTTTTAGCGTA-3' (for 102-site mutation)
[0055] Seq5: (forward) 5'-GCTAAAAAAGAATCCAACGAAGAC-3' (for 102-site mutation)
[0056] Seq6: (reverse) 5'-GGATCCTCAGGTGATACCCATCGGCAG-3'
[0057] A 240bp fragment was amplified by PCR with Seq3 and Seq4 primers, and a 200bp fragment was amplified by PCR with Seq5 and Seq6 primers. Then, the 240bp and 200bp fragments were mixed, and PCR amplification was carried out ...
example 3
[0059] Example 3: Construction and expression of the recombinant plasmid of the Δ23KGF (25(2) mutant
[0060] Δ23KGF (25C) is a variant in which the 25th tyrosine is mutated into cysteine based on the deletion of 23 amino acids at the N-terminal of human KGF. According to the method for example 2, synthesize following two pairs of primers:
[0061] Seq7; (Forward) 5'-CATATGTCTTGCGACTACATGGAAGGTGGTGAC
[0062] Seq8: (reverse) 5'-GGATCCTCAGGTGATAGCCATCGGCAG-3'
[0063] Using the artificially synthesized human KGF cDNA of Seq2 as a template, the fragment amplified by PCR was digested with Nde I and BamH I, and then inserted into the pET3a expression plasmid. After recombinant screening, transformation and expression screening, a strain expressing recombinant Δ23KGF (25C) was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com