DNA-cleaving antitumor agents
A compound, ethynyl technology, applied in the direction of antineoplastic drugs, antiviral agents, antibacterial drugs, etc., can solve the problem that heteroatom substitutes have not yet been widely developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] Detailed Description of Preferred Embodiments
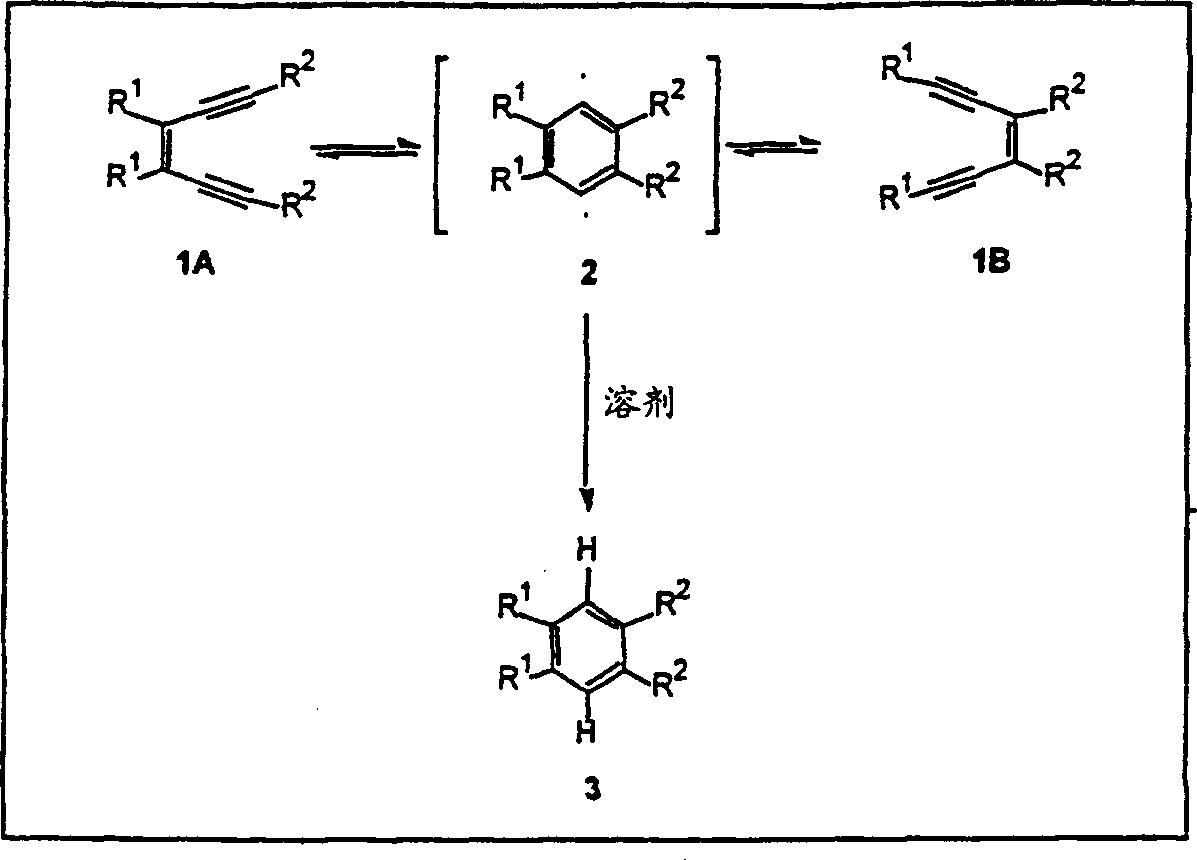
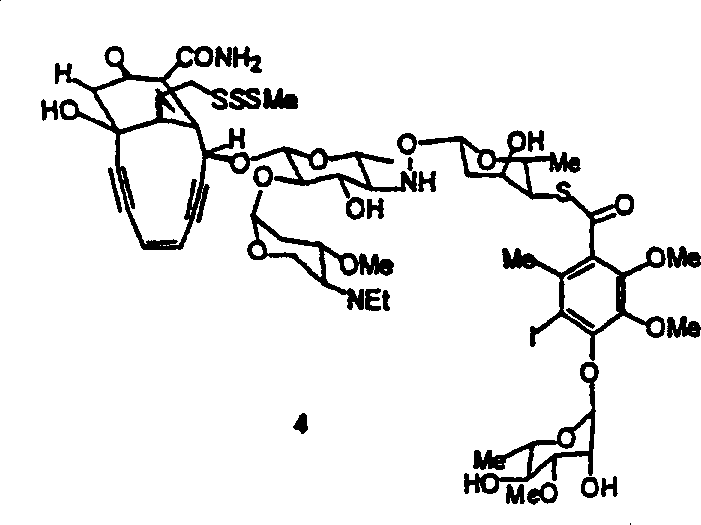
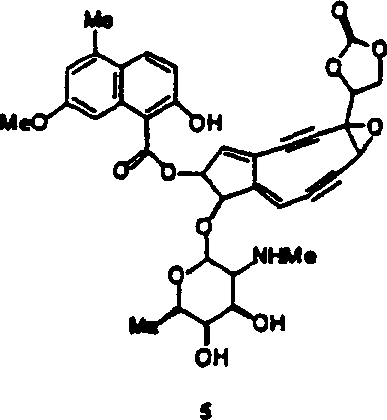
[0044] This article describes the synthesis and use of new azepine derivatives of enediynes, enediynes, and diallenes. Properly constructed aza derivatives can undergo thermal reactions to produce diradical intermediates. It is believed that the propensity of these aza-derivatives to undergo these diradical-generating reactions and of these diradical intermediates to undergo radical atom-abstracting reactions and other facilities leading to DNA strand scission The position and nature of the substituents on the alkyne, enyne propadiene or diallene. Aza-Bergman cyclizations have the advantage of being easier to perform than the corresponding Bergman cyclizations. Therefore, these aza-derivatives are capable of inducing DNA strand cleavage under physiological conditions.
[0045] In one embodiment, the aza-derivative has the formula A: where R 1 and R 4 Each is:
[0046] Substituted ethynyl, the structure is:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com