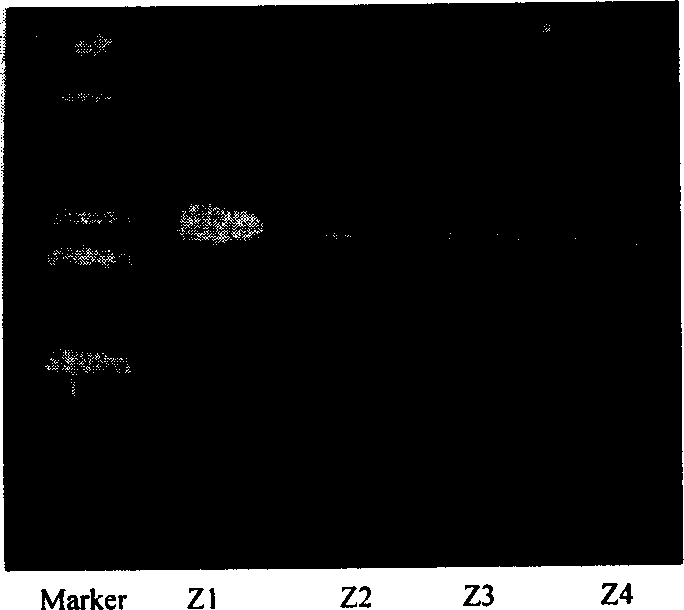3-deoxy-3-carbonyl-erycin lactone b and its engineeirng strain and application
A technology of saccharopolyspora mold and esters, applied in the field of macrolide compounds, can solve the problems of inability to overcome drug resistance, generation of drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Mass spectrometric identification of 3-deoxy-3-carbonyl-erythromycin lactone in fermentation broth: The fermentation broth of Saccharopolyspora M1 extracted by the above method was detected by ZabsPec mass spectrometer. Embodiment 1, construction of pWHM2201 plasmid
[0025]1. Design of PCR primers: In order to knock out the KR6 enzyme domain in the erythromycin synthesis gene, according to the literature CortesJ, Haydock SF, Roberts GA, et al. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. 1990, 348 (6297): 176-8 sequence, a fragment is amplified on each side of it, and there is a 20bp overlap between the fragments. The primers are: Fragment 1: Forward: 5’-TACGAATTCAGATCTCATGCGGCTCCTGGAGTCCGCAGTGGACG; Reverse: 5’-CTCGAGGTCGCCGGTGGCCGGGCCCGCCGGCTCCCCAGCTCGACTCG; Fragment 2: Forward: 5’-CGAGTCGAGCTGGGAGCCGGCGGGCTCCCGGCCACCGGCGACCTCGAGATCGTCCAGCCT; Reverse: 5’-TTCAAGCT.GCCT
[0026] Using f...
Embodiment 2
[0032] Example 2, PCR Identification of Chromosomal Integration and Chromosomal Secondary Recombination
[0033] 1) According to the sequence of the tsr gene (Hopwood DA, Bibb MJ, Chater KF et al. Genetic manipulation of stretomyces: laboratory manual. The John Innes Foundation, England, 1985), design and identify primers: Forward: 5'-CCTGAATTCATGACTGAGTTGGACACCATCGCA Reverse: 5- TCTAAGCTTGGAAACGTTGAGAACTCGGTCTG. The length of the amplified DNA fragment was 774bp.
[0034] 2) According to the erythromycin synthetic gene sequence, select PCR primers on both sides of the KR6 enzyme domain as follows:
[0035] Forward: 5'-AACGTCTTCCCGGCGGCACC;
[0036] Reverse: 5'-GTCGAGGTAGGACCGGACCC.
[0037] The length of the amplified fragment without KR6 removal was 1,770bp, and the length of the amplified DNA fragment with KR6 removal was 1,239bp.
[0038] 3) Homologous recombination of pWHM2201 plasmid and chromosome: After making protoplasts from Rhodomolgus Saccharopolyspora A226, th...
Embodiment 3
[0041] Embodiment 3, the screening of Saccharopolyspora erythraea (Saccharopolyspora erythraea) M
[0042] After the integrant Saccharopolyspora erythromycetes Z1 was continuously passed for two generations on the non-resistant R3M slant, the protoplasts were prepared and coated with the non-resistant R3M plate, and 40 colonies were picked from it and each was continuously coated with the Tsr-containing R3M slant and the Tsr-free plate. R3M bevel. Eight colonies (S erythraea M1-M8) grew only on the non-resistant R3M slant. Inoculate 4 colonies (M1-M4) for liquid culture to extract total DNA to remove KR6 identification primers for PCR amplification. The PCR product is about 1.2kb (such as image 3 shown), indicating that the KR6 enzyme domain on the chromosome has been knocked out. Saccharopolyspora erythraea M1 CGMCC №0604 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com