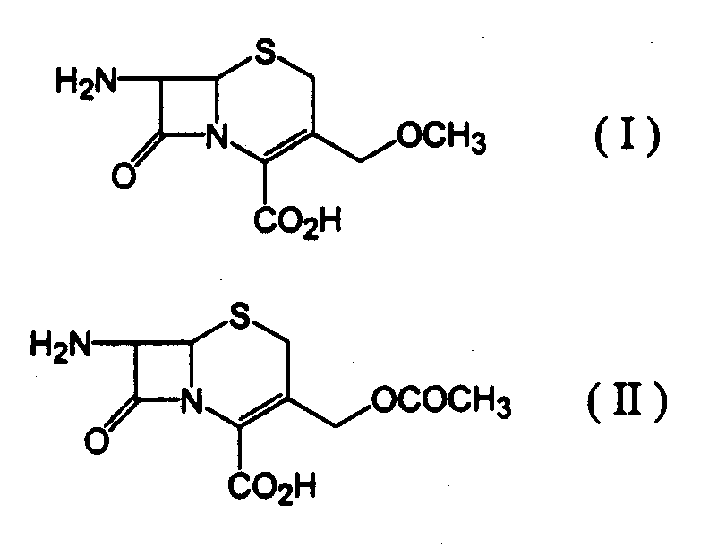
Process for preparing 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid
A technology of methoxymethyl and cephem, which is applied in the field of intermediates useful in the preparation of cephalosporin antibiotics, and can solve problems such as poor controllability of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 24.4 ml of methanesulfonic acid were mixed with 6.0 ml of an azeotropic mixture consisting of 70% trimethyl borate and 30% methanol, and the resulting mixture was cooled to 10°C. 10 g of 7-aminocephalosporanic acid was slowly added thereto and allowed to dissolve completely. Then, while the temperature was maintained at 10°C, an additional 6.3 ml of the trimethyl borate-methanol azeotrope was added over 1.5 hours and stirred for a further 1.5 hours. 35 ml of cold water was added dropwise to the resulting mixture, and then 15.6 g of sodium carbonate was carefully added thereto in a small amount, followed by dropwise addition of 100 ml of acetone.
[0026] The resulting mixture was filtered to remove solid sodium methanesulfonate and the filtrate was cooled to 5°C. A solution containing 11.5 g of sodium carbonate dissolved in 40 ml of water was added dropwise to the filtrate over 1 hour to adjust the pH to 3.2. The formed solid was filtered, washed with water and aceton...
Embodiment 2
[0029] 26.8 ml of methanesulfonic acid were mixed with 5.0 ml of an azeotropic mixture consisting of 70% trimethyl borate and 30% methanol, and the resulting mixture was cooled to 10°C. 10 g of 7-aminocephalosporanic acid was slowly added thereto and allowed to dissolve completely. Then, while the temperature was maintained at 10°C, an additional 10.2 ml of the trimethyl borate-methanol azeotrope was added over 1.5 hours, and the mixture was stirred for a further 2 hours. 30 ml of cold water was added dropwise to the resulting mixture, then 15.6 g of sodium carbonate was carefully added thereto in a small amount, and 100 ml of acetone was added dropwise thereto.
[0030] The resulting mixture was filtered to remove solid sodium methanesulfonate and the filtrate was cooled to 5°C. A solution containing 13.9 g of sodium carbonate dissolved in 50 ml of water was added dropwise to the filtrate over 1 hour to adjust the pH to 3.5. The formed solid was filtered, washed with water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com