Method for separating and recovery difluorochloromethane azeotropic hexafluoropropene
A technology of difluorochloromethane and hexafluoropropylene, which is applied in the field of novel separation and recovery of difluorochloromethane-hexafluoropropylene azeotrope, and can solve problems such as increased operating costs, complicated process flow, and high price. Achieve the effect of lower operating cost, simple process flow and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
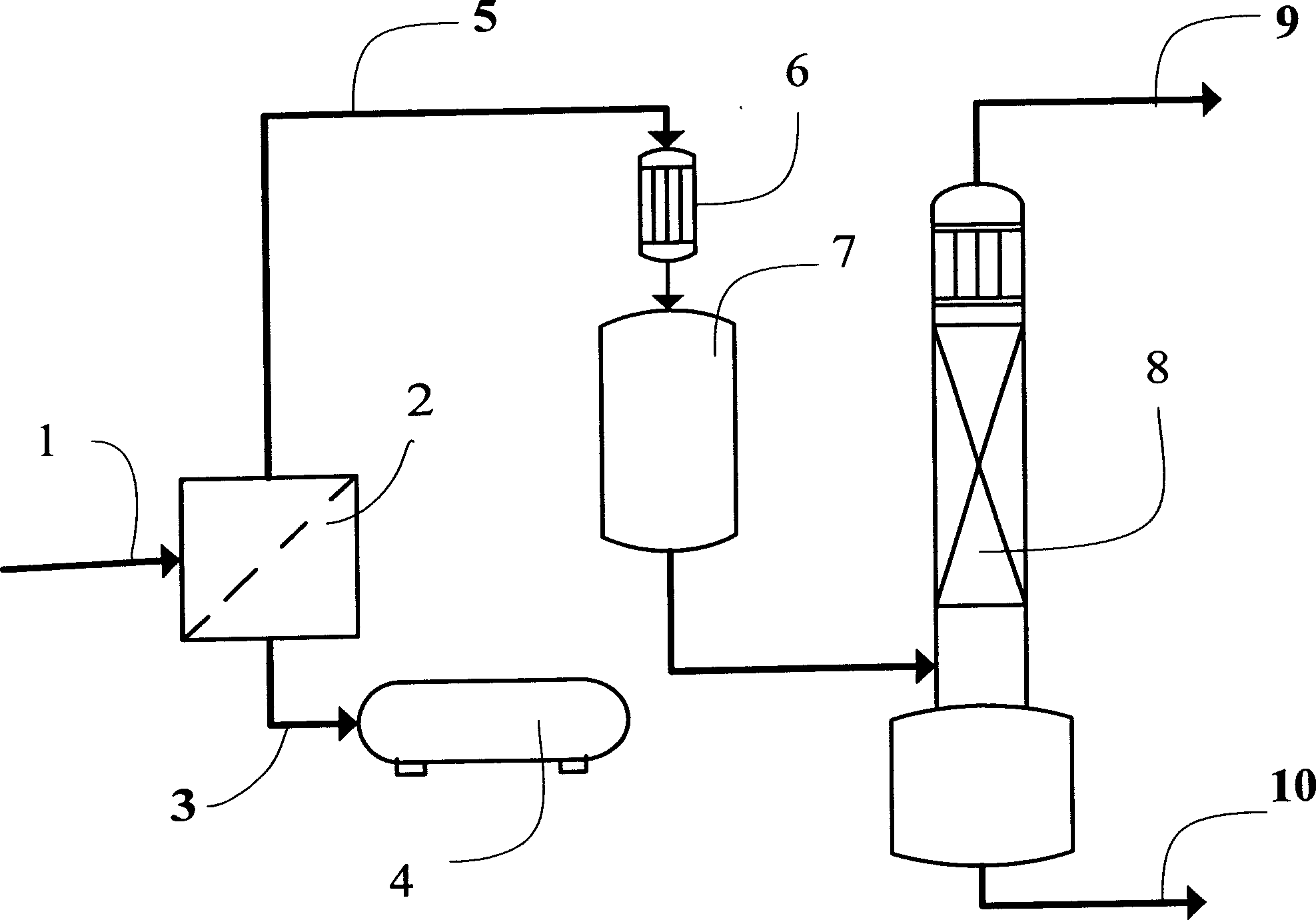
[0050] Please combine figure 1 , at an ambient temperature of 20°C and a pressure of 0.5 MPa (absolute pressure), the azeotropic mixture of difluorochloromethane-hexafluoropropylene (the amount of the substance consists of 87% of difluorochloromethane and 13% of hexafluoropropylene) passes through Pipeline 1 passes through gas separation membrane module 2 in a gaseous state, and two streams of gas whose azeotropic ratio has been destroyed are obtained from gas separation membrane module 2: one is the permeate gas (substance) mainly composed of difluorochloromethane (HCFC-22) The amount consists of difluorochloromethane 97.67%, hexafluoropropylene 2.33%), which is introduced into the TFE production plant low-pressure gas cabinet 4 through pipeline 3 and reclaimed; the other is the retentate gas based on hexafluoropropylene (HFP) ( The amount of substance consists of chlorodifluoromethane 14.2%, hexafluoropropylene 85.8%).
[0051] The retentate gas is condensed through the pi...
Embodiment 2
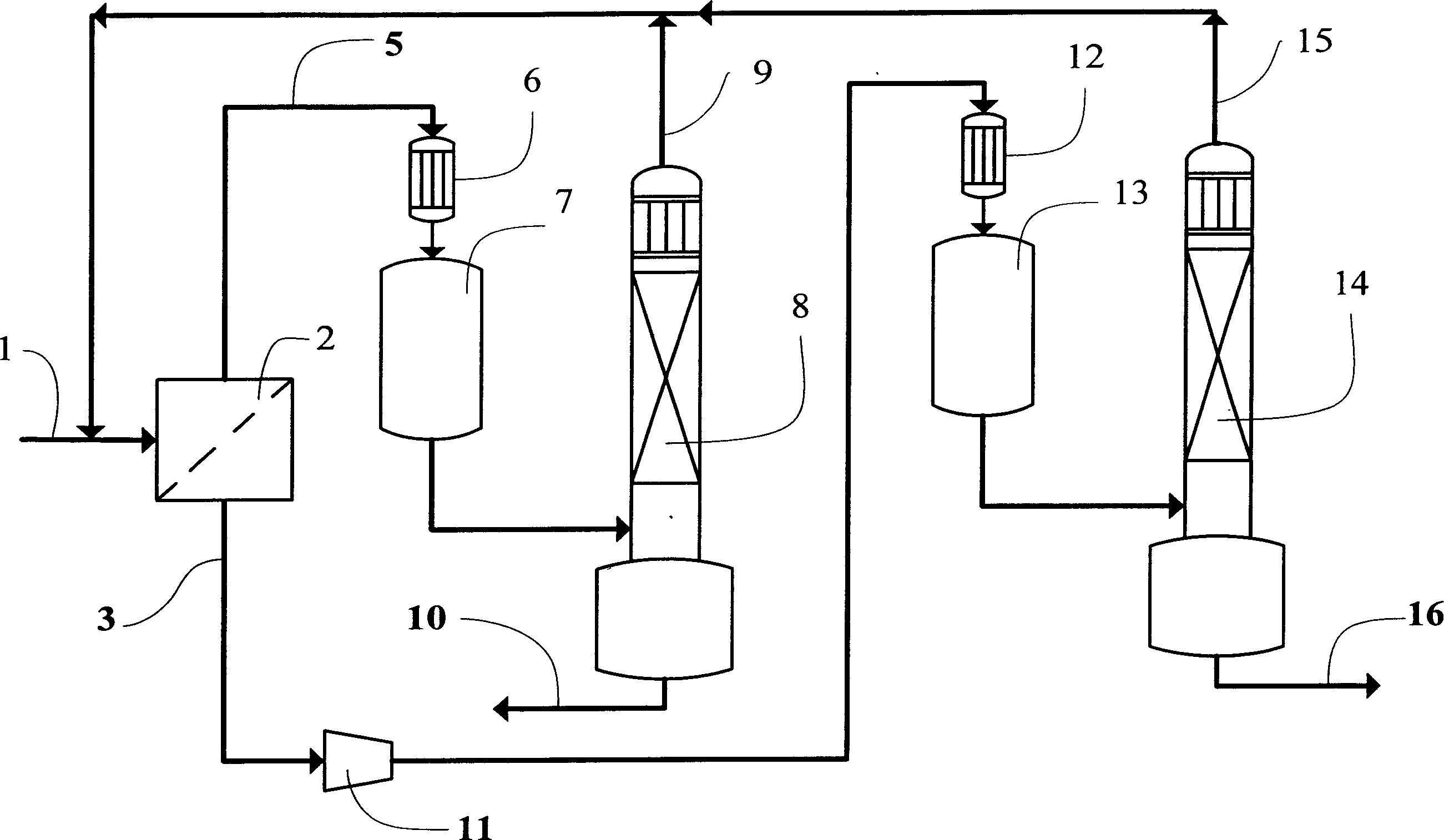
[0053] Please combine figure 2 , at an ambient temperature of 20°C and a pressure of 0.5 MPa (absolute pressure), the azeotropic mixture of difluorochloromethane-hexafluoropropylene (the amount of the substance consists of 87% of difluorochloromethane and 13% of hexafluoropropylene) passes through Pipeline 1 passes through gas separation membrane module 2 in a gaseous state, and two streams of gas whose azeotropic ratio has been destroyed are obtained from gas separation membrane module 2: one is the permeate gas (substance) mainly composed of difluorochloromethane (HCFC-22) The amount composition is 97.67% of difluorochloromethane, 2.33% of hexafluoropropylene); the other is the retentate gas mainly based on hexafluoropropylene (HFP) (the amount of substance is composed of 14.2% of difluorochloromethane, Hexafluoropropylene 85.8%).
[0054] The retentate gas is condensed through the pipeline 5 through the -35°C condenser 6, collected into the condensate collection tank 7, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com