Method for synthesizing polymeric azo dyes
A polymer, azo-based technology, applied in the coupling reaction of azo dyes, azo dyes, organic dyes, etc., can solve problems such as difficulty and expensive impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0077] With stirring, to a 5 L, cooling jacketed, round bottom flask was added sequentially: 4-Nitroaniline (2.5 moles, 345 g), water (2.0 L), and hydrochloric acid (37%, 0.5 L) . The mixture was cooled to 0-10 °C and pre-cooled IBN (15 °C, 2.6 moles, 268 g) was added to the flask. At this point the diazotization reaction will occur immediately. After the IBN addition was complete, the reaction mixture was stirred for an additional hour. The diazonium salt formed is then ready for the sequential azo coupling reaction.
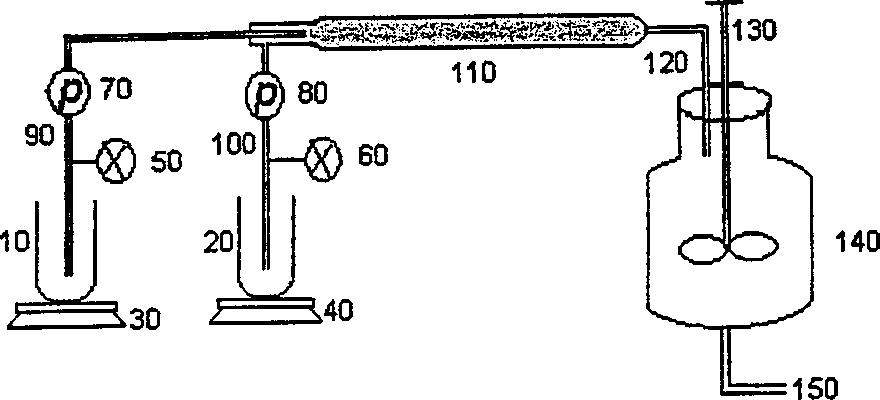
[0078] Into a 20 L glass reactor, add PHS-MMA (55-45 molar ratio, 2.5 moles, 300 g), THF (2.5 L), methanol (3.0 L), and tetramethylammonium hydroxide (TMAH, 25 %, 2.5 liters), and the mixed solution was cooled to 0-10°C. The azo coupling reaction was performed by pumping the diazonium salt solution and the PHS solution simultaneously through a 1 / 4 inch by 60 inch in-line static mixer (from Cole-Parmer Instrument Co.). Two gear pumps (70 and 80 in Figure 1)...
Embodiment 4
[0080] To a 5 L, cooled jacketed, round bottom flask were added sequentially with stirring: aminoacetanilide (AAA, 2.5 moles, 375.0 g), water (1.5 L), and hydrochloric acid (37%, 0.5 L) . The suspension was cooled to 0-5 °C. Pre-cooled IBN (15°C, 2.55 moles, 262.6 grams) was added to the flask. When the AAA reacts, the newly formed diazonium salt dissolves in water, giving a brown solution.
[0081]In a 20-liter reactor at 5°C, PHS-MMA (55-45 molar ratio, 2.3 moles, 506 g) was dissolved in THF (4.9 liters), water (1.3 liters), methanol (2.5 liters), and in a mixture of TMAH (25%, 1.9 L). The diazonium salt solution and the PHS solution were pumped at a flow ratio of 1:4.2 between the diazonium salt solution and the PHAS-MMA solution through a 1 / 4 inch x 60 inch in-line static mixer connected to a centrifugal pump. Monitoring is performed using flow gravimetric analysis. The contact time of the reactants was about 2 seconds. It took approximately 50 minutes to pump all th...
Embodiment 5
[0083] 2-(Methacryloyloxy)ethylacetoacetate (MEAA, 2.5 moles, 535.0 grams) and THF (4.0 L) were added to a 10 L round bottom flask. The solution was degassed by bubbling nitrogen gas through the solution for 1 hour. Then, a solution of methyl methacrylate (MMA, 2.5 mol, 250.0 g) and 2,2'-azobisisobutyronitrile (AIBN, 41.0 g) in THF was injected into the first solution. The mixture was further degassed with nitrogen for 30 minutes. The nitrogen vent needle was then removed and the mixture was stirred overnight at 65°C in a sealed container. The solution was then precipitated into 25 liters of 2-propanol. The polymer product (MEAA-MMA, 50-50 molar ratio) was collected as a yellow solid in 96% yield. GPC showed that the weight average molecular weight Mw of the polymer was 32800.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com