Cytotoxin T lymphocyte
A lymphocyte and cytotoxic technology, applied in the field of treatment and diagnosis, can solve the problems of backward analysis and research, CTL that is difficult to provide tumor treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
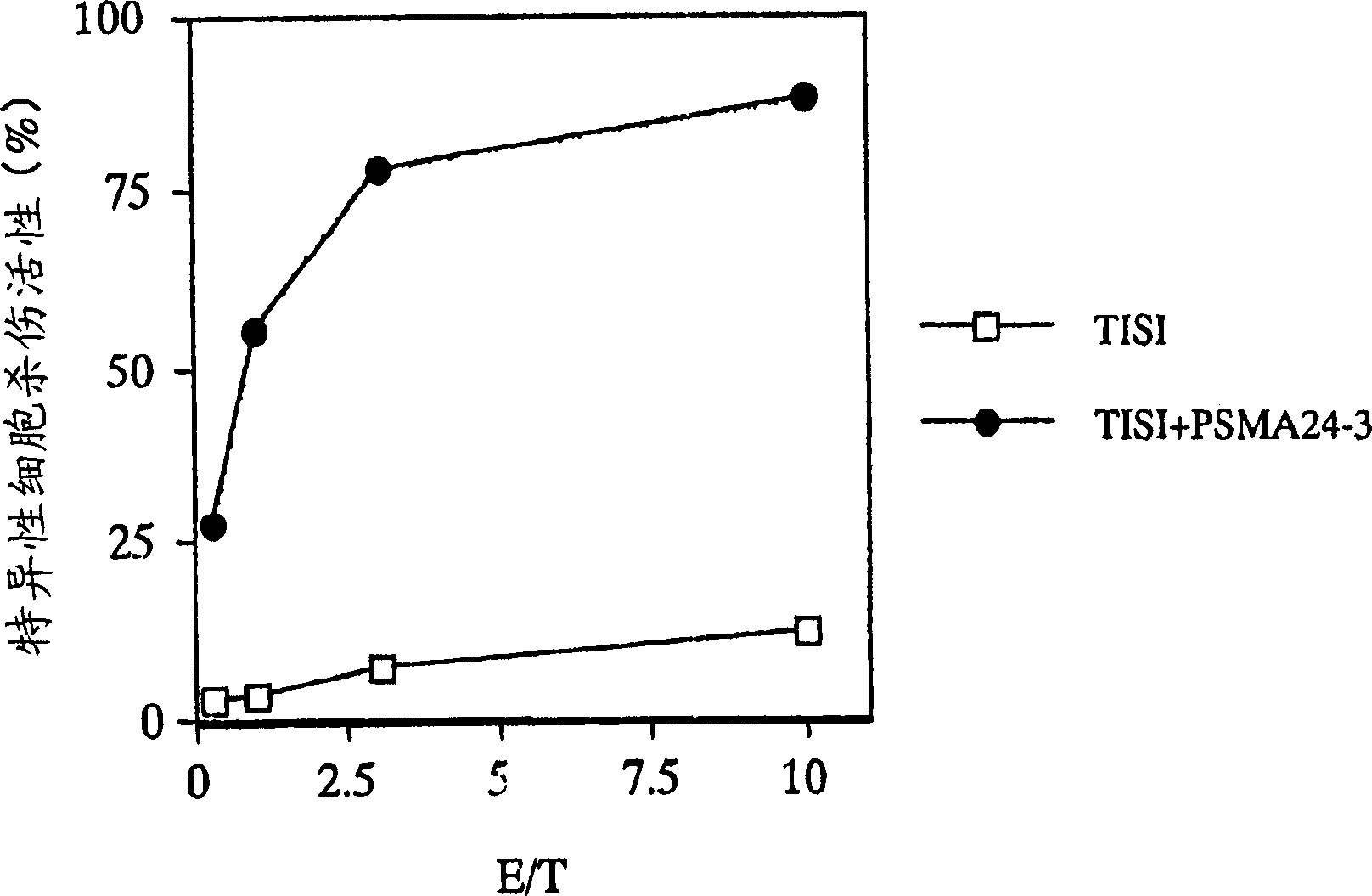
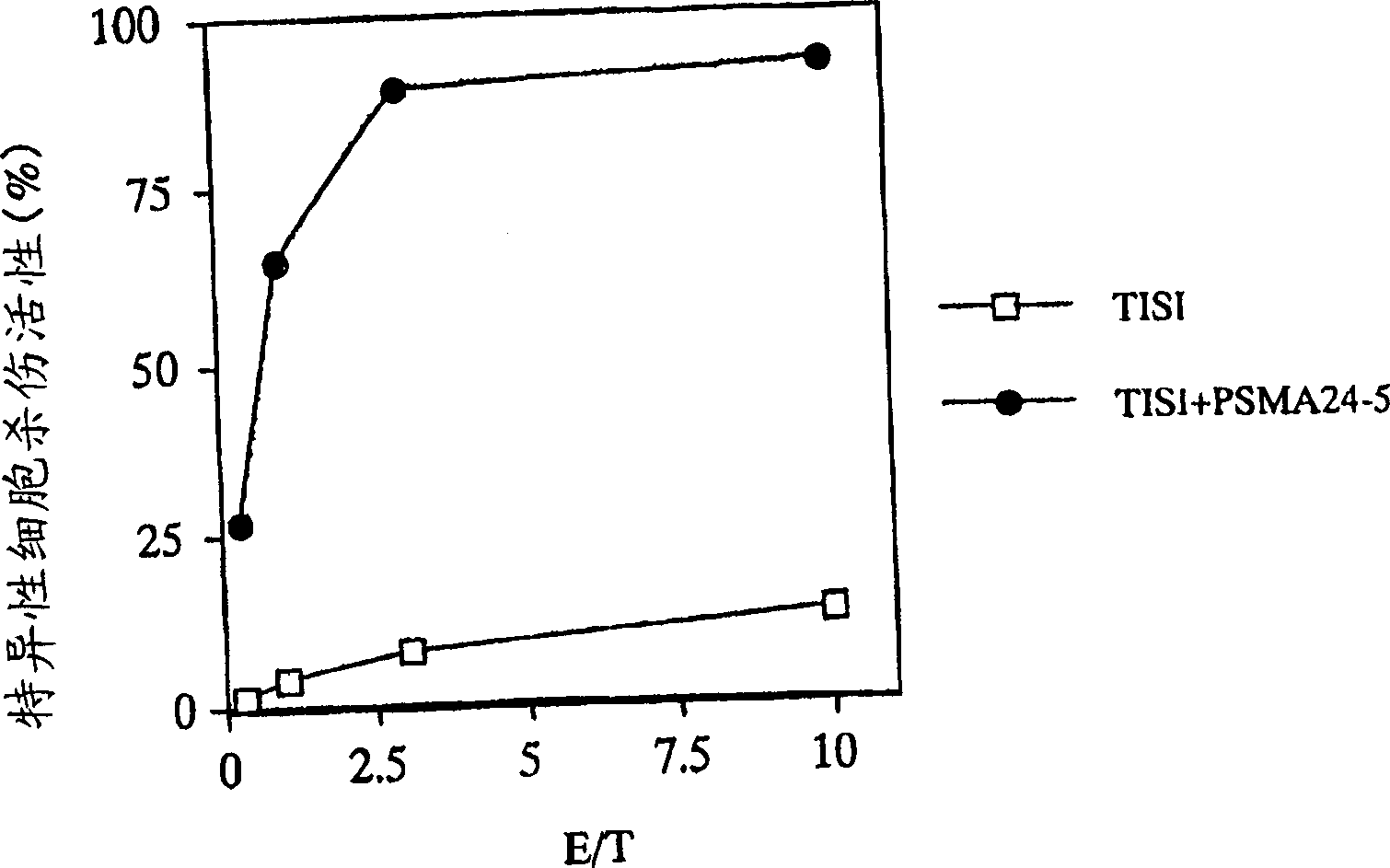
[0144] Example 1 Preparation of PSMA Antigen Peptide-Specific CTL
[0145] In this example, CTLs were prepared by the induction method using dendritic cells (DC) for antigen presentation.
[0146] (1) Screening and synthesis of peptides for CTL induction
[0147] For the amino acid sequence of the PSMA protein composed of 750 amino acids, the peptide with the HLA-A24 binding motif structure (the second amino acid residue at the N-terminal is selected from Tyr, Phe, Trp and Met, and the amino acid residue at the C-terminal Selected from Leu, Ile, Phe and Trp) as the center, screen 9 peptides shown in Table 1 as candidate peptides of PSMA antigenic peptides.
[0148] serial number
Position in PSMA
name
10
298-306
PSMA24-1
11
624-632
PSMA24-2
1
227-235
PSMA24-3
12
606-614
PSMA24-4
2
178-186
PSMA24-5
13
74-83
PSMA24-6
14
565-574
PSMA24-7
...
Embodiment 2
[0173] Example 2 Preparation of PSMA Antigenic Peptide Functional Derivatives
[0174] (1) Synthesis of PSMA24-5 variants
[0175] serial number
name
8
PSMA24-5-1A
17
PSMA24-5-4A
9
PSMA24-5-5A
18
PSMA24-5-6A
19
PSMA24-5-7A
20
PSMA24-5-8A
5
PSMA24-5-9L
[0176] A total of seven peptides, PSMA24-5-1A to PSMA24-5-9L shown in Table 2, were prepared with a peptide synthesizer.
[0177] (2) Identification of functional derivatives of PSMA24-5
[0178] It was checked whether the CTL obtained in Example 1 above, which can recognize the complex of PSMA24-5 and HLA-A24 molecule, can recognize the complex of the peptide shown in Table 2 and HLA-A24 molecule.
[0179] First, for the CTL that can recognize PSMA24-5 prepared by the same method as in Example 1 (2), for 51 Cr-labeled TISI (-) cells, and 7 kinds of peptides selected from PSMA24-5-1A~PSMA24-5-9L and p...
Embodiment 3
[0181] Example 3 Using adenovirus to evaluate CTL
[0182] (1) Preparation of genes from adenovirus into target cells
[0183] As target cells endogenously presenting antigens, tumor cells expressing HLA-A24 and PSMA were constructed. That is, for the prostate cancer cell line LNCaP cells expressing PSMA but not expressing HLA-A24, the gene was introduced into the cells with an HLA-A24 gene-transforming adenovirus vector. For cancer cells MKN45, 888mel, SW480, and T.T that express HLA-A24 but do not express PSMA, the PSMA gene is introduced into the cells with an adenoviral vector that transduces the PSMA gene.
[0184] The transgenic adenoviral vector was prepared according to the COS-TPC method.
[0185] First, place the 5×10 5 4ml of cell suspension per ml was inoculated in a 6-well cell culture plate and cultured overnight. After culturing, the above cells were infected with HLA-A24 gene-transformed adenovirus, PSMA gene-transformed adenovirus or non-transformed adenov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com