Preparation process of spinal cord and peripheral nerve repairing material
A technology for peripheral nerves and repair materials, applied in catheters and other directions, can solve problems such as chaotic and irregular diameters of tubes and directions of canal gaps, inability of regenerative fibers to grow in an effective direction, and effects on treatment or experiment effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
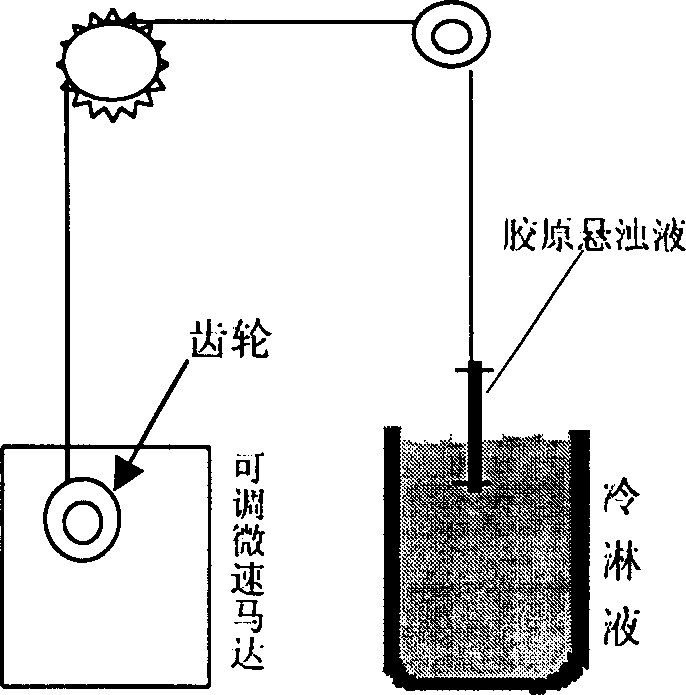
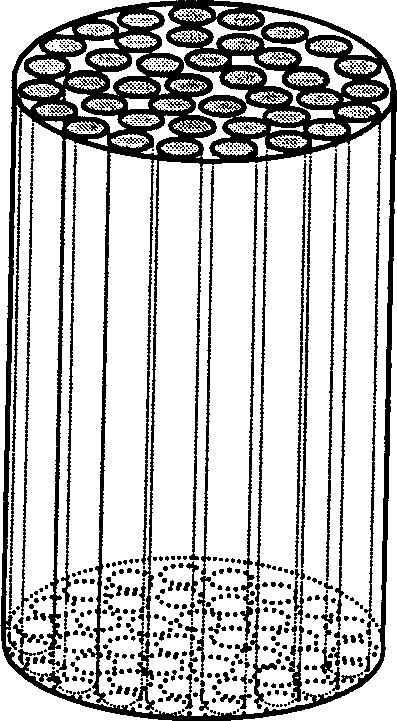
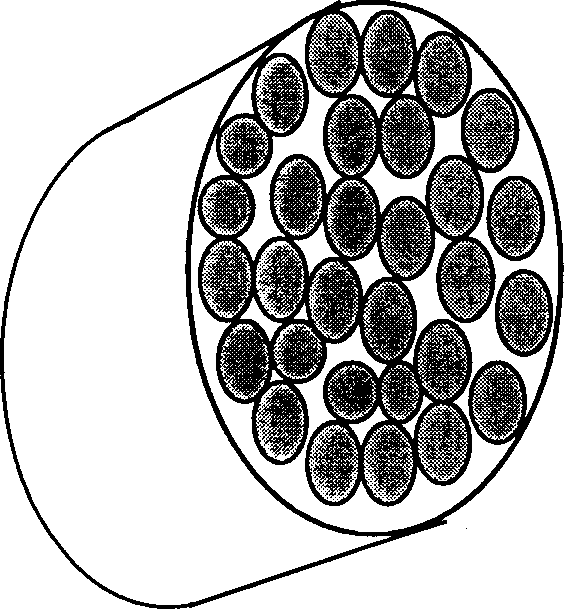
[0021] Embodiment 1: see figure 1 , according to the technical scheme of the present invention, taking type I collagen and chondroitin sulfate-6 as an example, its preparation method is carried out according to the following steps:
[0022] 1) During production, dissolve type I collagen at 27.5mg / ml in 0.05M (acetic acid, hydrochloric acid, etc., the same below) acidic solution, and stir at 2000r / min for 90min at a constant temperature of 4°C to 10°C , made into a suspension;
[0023] 2) Add chondroitin sulfate-6 at 1.33mg / ml into 0.05M acidic solution, and stir at 2000r / min for 90min at a constant temperature of 4°C to 10°C to make a suspension;
[0024] 3) Mix the two suspensions at a ratio of 5:1, and continue to stir at 2000r / min for 90 minutes at 4°C to 10°C to make a mixed suspension of collagen and chondroitin sulfate-6, and make the suspension The solution was placed in a long-necked flask, vacuumed and left for 30 minutes to eliminate foam;
[0025] 4) Inject the m...
example 2~6
[0042] The following examples 2-6. The mixing ratio of collagen (type I, III, IV) and aminoglycan (chondroitin sulfate-6 or chondroitin sulfate-4) is the same.
Embodiment 2
[0043] Example 2: Prepared by using type III collagen and chondroitin sulfate-6 as raw materials. Its implementation process is with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com