Layered arrangements of lithium cells
A battery and stack technology, applied in the field of negative electrodes, can solve the problem of lack of effective mechanism to protect lithium negative electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
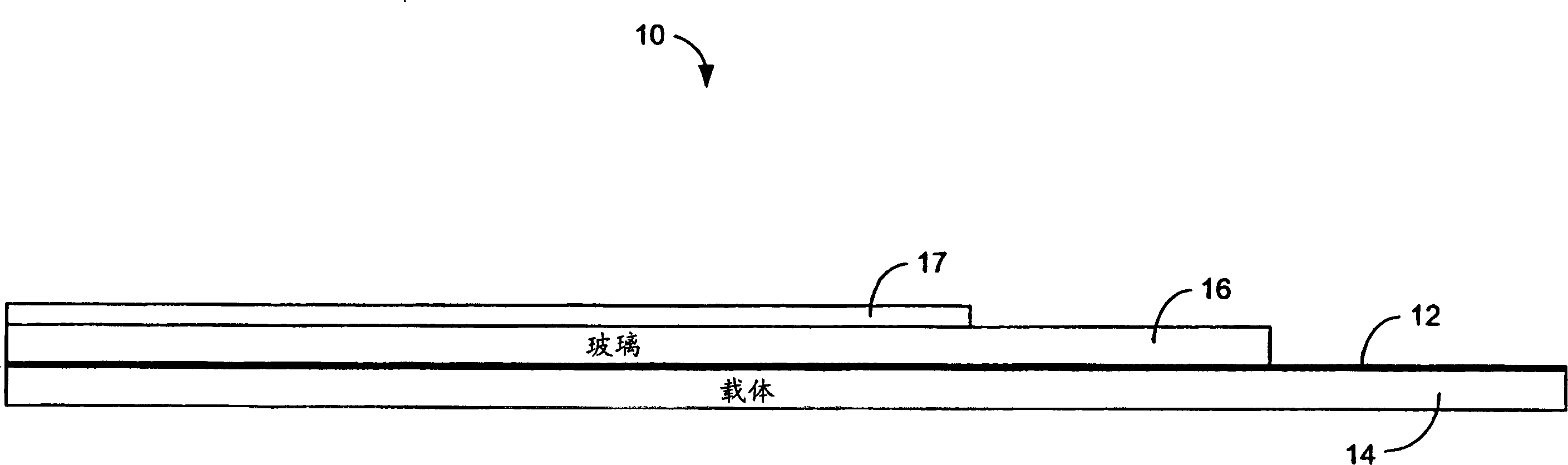
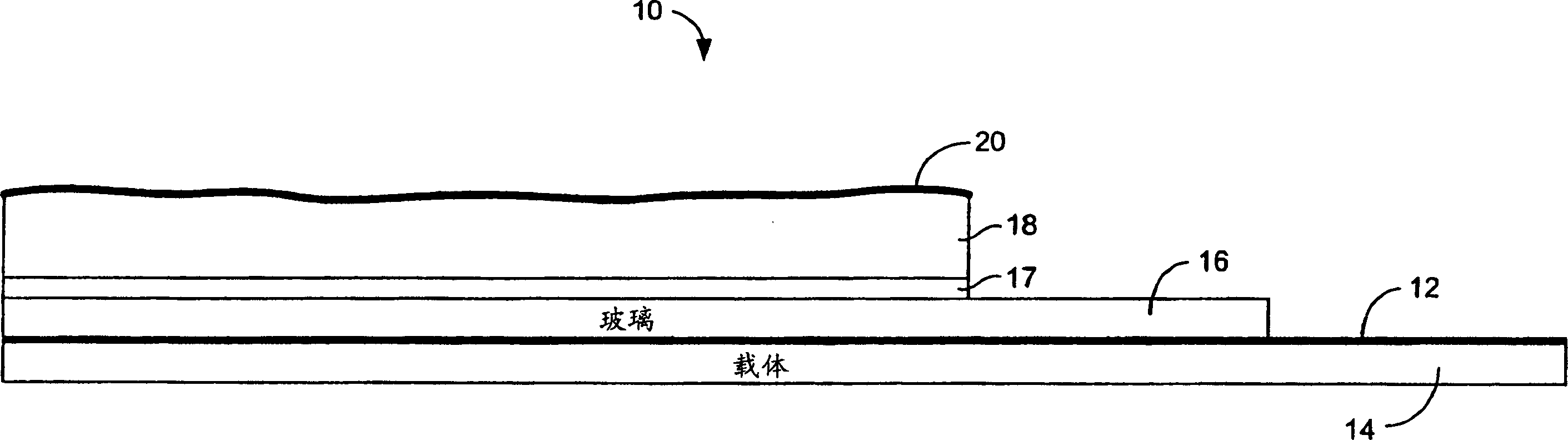
[0138] LiPON / Kynar / PET samples were bonded on lithium foil. First, Kynar is pressed onto a PET carrier. A LiPON barrier layer was then sputtered on top of the Kynar layer. The thicknesses of the resulting LiPON, Kynar, and PET were about 200 nm, 4 µm, and 25 µm, respectively. The sample was transferred to an argon-filled glove box. Lithium foil with a thickness of 125 μm purchased from Argo-Tech (Canada) was pressed onto a stainless steel current collector and then merged with the LiPON / Kynar / PET structure so that the lithium and LiPON surfaces were in contact with each other. The above membranes were placed between two glass plates, clamped, and stored at room temperature for about two days. After preservation, the structure is loosened and converted into a PET layer on top. Then try peeling off the PET layer with tweezers. Because of the minimal adhesion between LiPON and lithium, it is difficult to peel off the PET support while maintaining the contact between LiPON an...
Embodiment 2
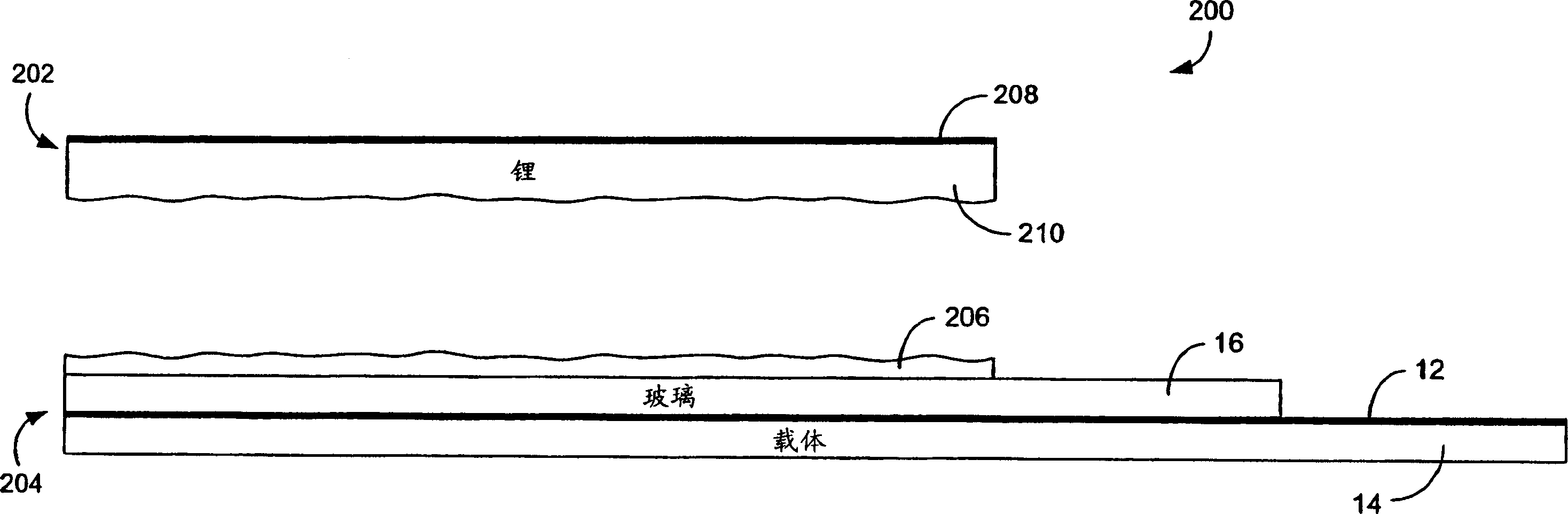
[0140] A thin aluminum film approximately 150 nanometers (0.15 microns) thick was evaporated onto a LiPON / Kynar / PET sample prepared as described in Example 1. The resulting sample was transferred to an argon-filled glove box. Lithium foils purchased from Argo-Tech (Canada) with a thickness of 125 μm were pressed onto stainless steel current collectors and then merged with Al / LiPON / Kynar / PET structures so that the lithium and aluminum surfaces were in contact with each other. The above membrane was placed between two glass plates, clamped, and stored at room temperature. The formation of lithium-aluminum alloys can be monitored through transparent PET / Kynar / LiPON samples. Because the light reflectance of the smooth aluminum layer is very high, while the light reflectance of the LiAl alloy is much lower, a decrease in reflectance corresponding to the alloy formation is observed. The formation of a low-reflectance gray lithium-aluminum alloy was then immediately observed, and a...
Embodiment 3
[0142] Except for bonding at room temperature for 1 hour, other steps are the same as in Example 2. No further storage at 55°C. Thus, the bonding procedure was the same as that of Example 1, except that no aluminum bonding layer was used. The resulting adhesion was not as good as Example 2, but much better than Example 1 (no aluminum).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com