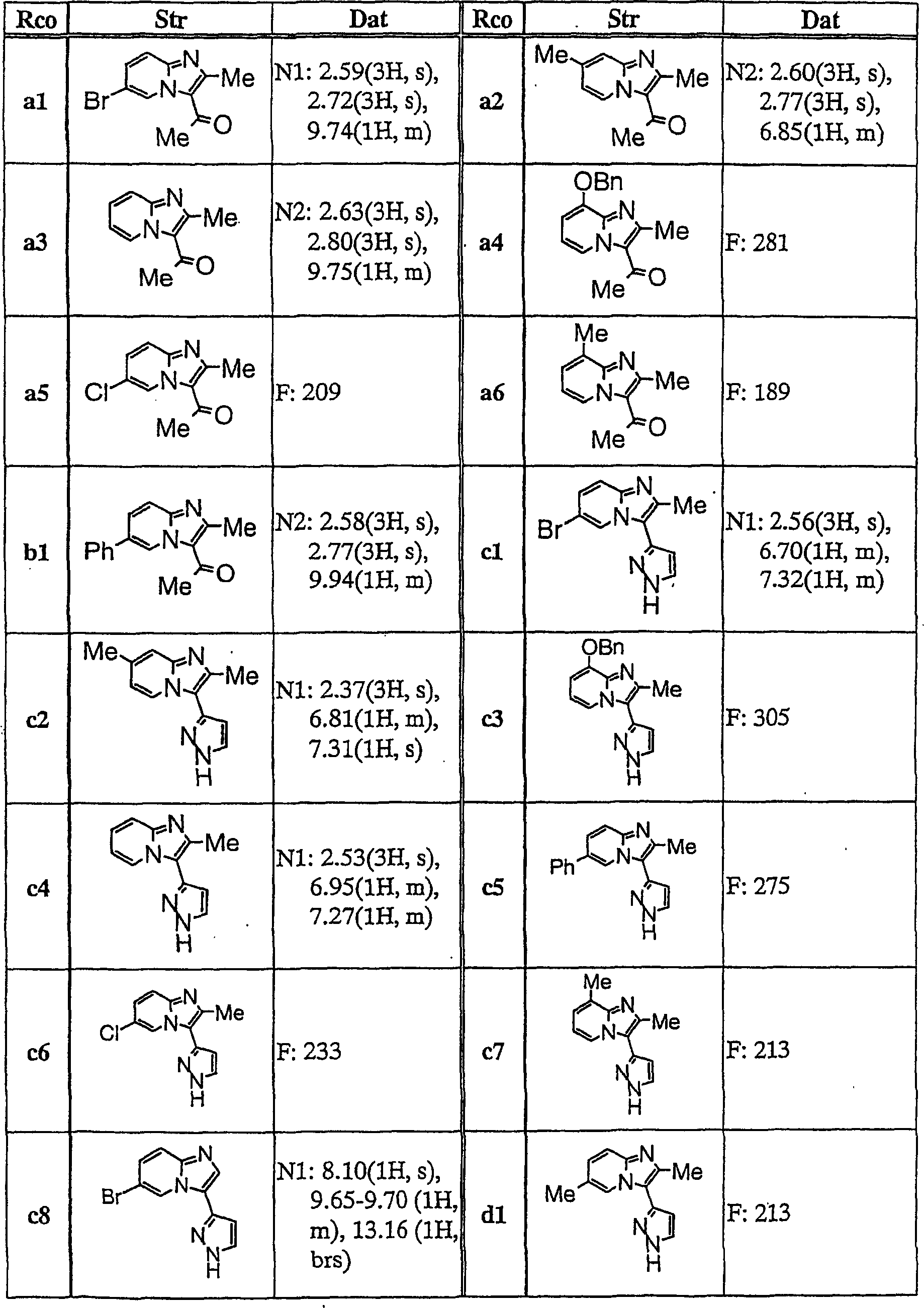
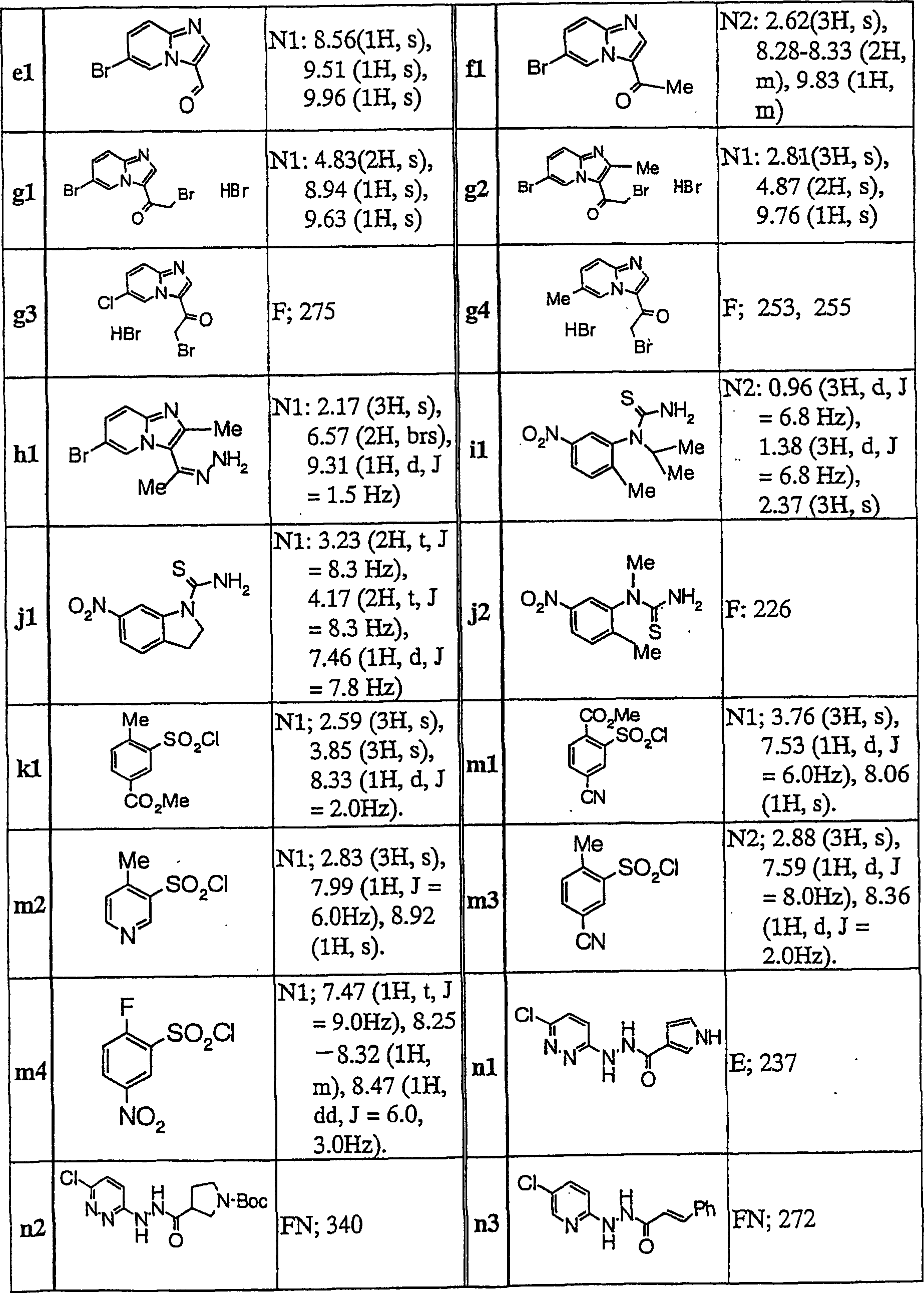
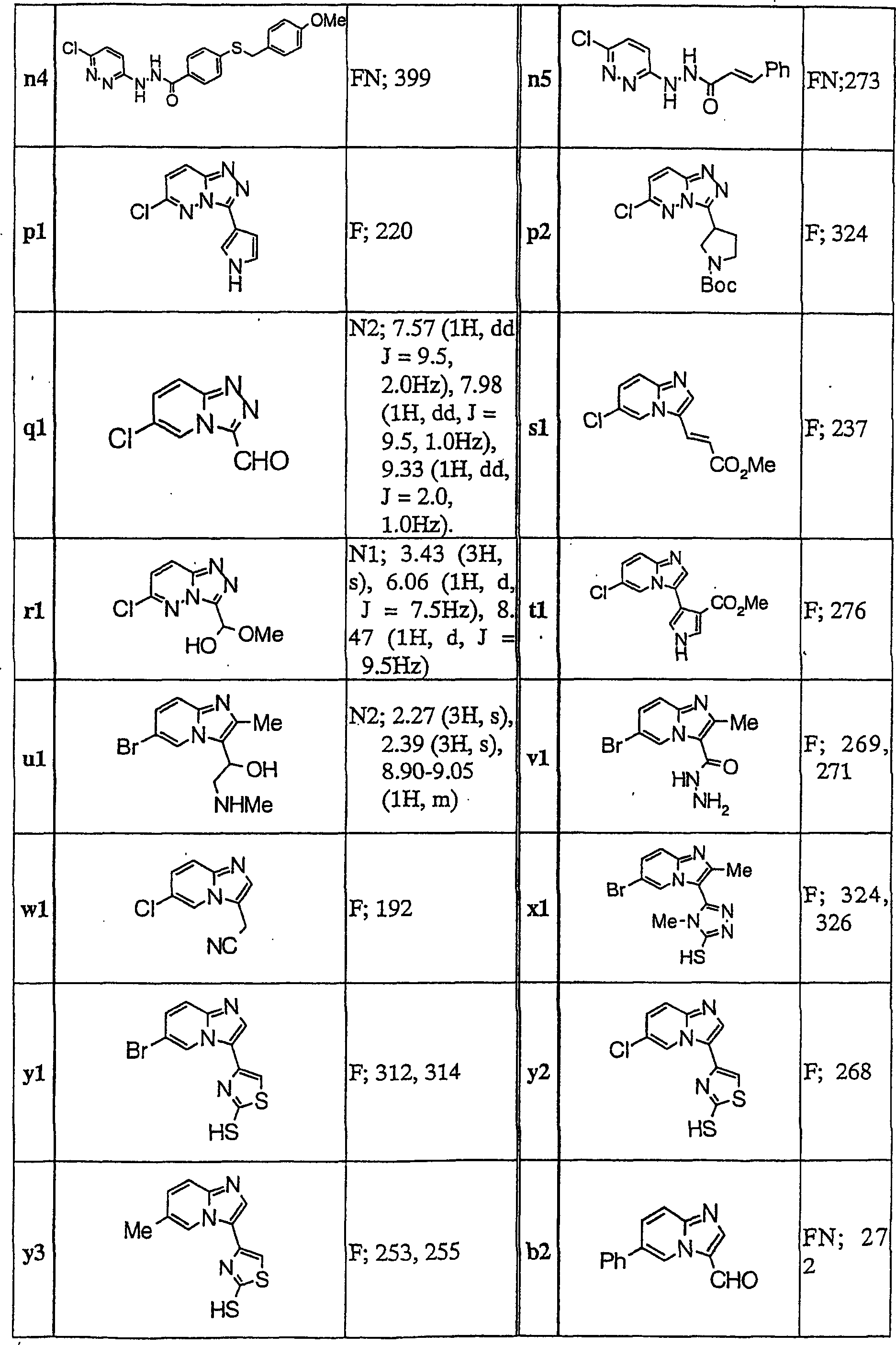
Imidazopyridine derivatives
A technology of imidazopyridine and derivatives, applied in the field of imidazopyridine derivatives, can solve the problems of different structures, no description, no disclosure of inhibitory activity and anticancer effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] To a mixture of compound c4 (0.10 g) and pyridine (2.5 ml), benzenesulfonyl chloride (0.10 g) was added, followed by stirring at 100°C for 4 hours. After concentrating the reaction solution under reduced pressure, the resultant was extracted with ethyl acetate, and the extract was washed with saturated aqueous sodium bicarbonate solution and dried over anhydrous magnesium sulfate. The organic layer was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (eluent; chloroform:methanol=100:0-99:1) to obtain Compound 1 (0.11 g).
Embodiment 2
[0155] To 2-methyl-5-nitrobenzoic acid (500 mg) was added thionyl chloride (5 mL). After the reaction mixture was heated to reflux for 1 hour, it was concentrated under reduced pressure and dried in vacuo. To the obtained solid were added THF (10 ml), chloroform (10 ml), TEA (2 g), and compound c1 (440 mg), followed by stirring at room temperature for 3 hours. It was diluted with chloroform, washed with water and saturated aqueous sodium bicarbonate solution. The obtained organic layer was dried with anhydrous magnesium sulfate, and dried under reduced pressure. The resulting solid was purified by silica gel column chromatography (eluent; chloroform:methanol=200:1) to obtain Compound 31 (72 mg).
Embodiment 3
[0157] To compound h1 (1.2 g) were added pyridine (10 ml) and 5-nitro-2-methylbenzenesulfonyl chloride (1.1 g) at room temperature, and stirred for 5 hours. The reaction solution was concentrated under reduced pressure, and the residue was diluted with chloroform and washed with water. The obtained organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The resulting residue was crystallized from chloroform, and crude crystals were collected by filtration. The resulting crude crystals were washed with hot methanol to obtain Compound 32 (1.47 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com