Fluoric alpha-aminoalkyl phosphonate with agricultural antiviral activity and its synthesis
A technology of aminoalkylphosphonate and trifluoromethyl, which is applied in the field of fluorine-containing α-aminoalkylphosphonate with agricultural anti-plant virus activity and the field of synthesis thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
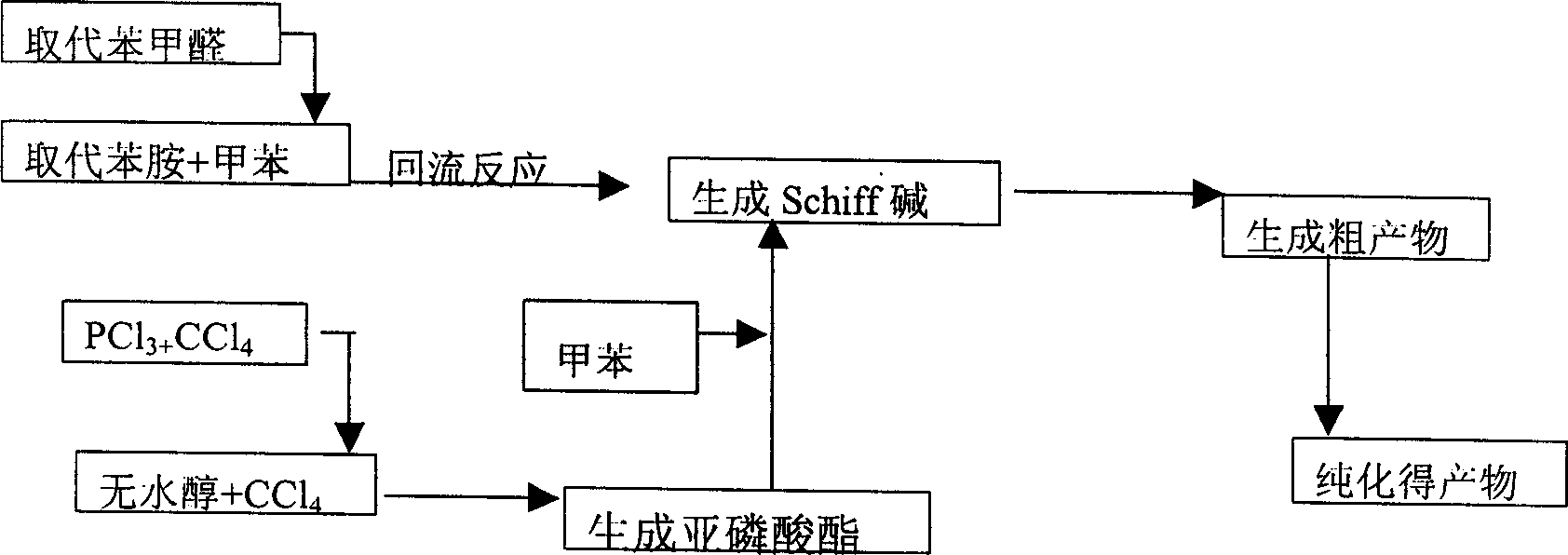
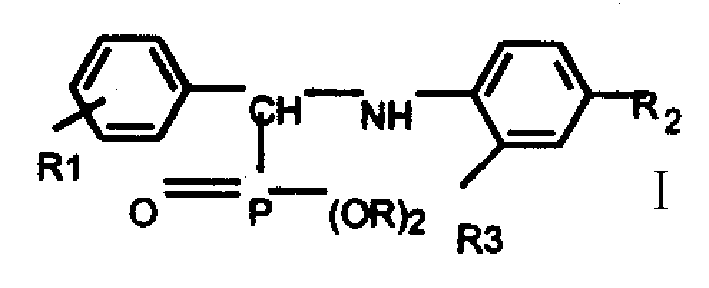
[0063] Embodiment (1): The synthesis (I) of compound e is in the 25mL there-necked flask that water separation reflux device and thermometer are housed, add p-trifluoromethylaniline 5.3mmol, 15mL toluene, after stirring and dissolving, add 5.3mmol p-trifluoromethylaniline Chlorobenzaldehyde, heated and refluxed for 1h, cooled to room temperature, the solution was turbid and strong, evaporated toluene, and recrystallized three times with ethanol to obtain a light yellow solid.
[0064] (II), O, the synthesis of O-diethyl phosphite is in the 500ml four-neck bottle that drying tube, nitrogen protection device, gas absorption device and constant pressure funnel are housed, add 75ml carbon tetrachloride and 1.8mol nitric acid Water and ethanol, under ice-water bath cooling, slowly add 0.51mol PCl3 dissolved in 50ml carbon tetrachloride dropwise, control the dropwise addition in 1.5-2 hours; slowly raise the temperature to 70°C and react for 1 hour, the solution changes from colorles...
Embodiment (2
[0066] Embodiment (2): the synthesis (I) of compound f is in the 25mL there-necked flask that water separation reflux device and thermometer are housed, add p-trifluoromethylaniline 5.3mmol, 15mL toluene, after stirring and dissolving, add 5.3mmol o Fluorobenzaldehyde, heated and refluxed for 1.5h, cooled to room temperature, the solution was turbid and strong, evaporated toluene, and recrystallized three times with ethanol to obtain a light yellow solid.
[0067] (II), O, the synthesis of O-diethyl phosphite is in the 500ml four-neck bottle that drying tube, gas absorption device, nitrogen protection device and constant pressure funnel are housed, add 75ml carbon tetrachloride and 1.8mol nitric acid Water and ethanol, under ice-water bath cooling, slowly add 0.51mol PCl3 dissolved in 50ml carbon tetrachloride dropwise, control the dropwise addition in 1.5-2 hours; slowly raise the temperature to 70°C and react for 1 hour, the solution changes from colorless to light Yellow, u...
Embodiment (3
[0069] Embodiment (3): The synthesis (I) of compound c is in the 25mL there-necked flask that water separation reflux device and thermometer are housed, add p-trifluoromethylaniline 5.3mmol, 15mL toluene, after stirring and dissolving, add 5.3mmol p-trifluoromethylaniline Fluorobenzaldehyde, heated and refluxed for 1h, cooled to room temperature, the solution was turbid and strong, evaporated toluene, and recrystallized three times with ethanol to obtain a light yellow solid.
[0070](II), O, the synthesis of O-diisopropyl phosphite In the 500ml four-necked bottle that drying tube, gas absorption device and constant pressure funnel are housed, add 75ml carbon tetrachloride and 1.8mol anhydrous isopropyl Alcohol, under cooling in an ice-water bath, slowly add 0.51mol PCl3 dissolved in 50ml of carbon tetrachloride dropwise, and control the dropwise addition in 1.5 hours; slowly raise the temperature to 70°C for 1 hour, and the solution changes from colorless to light yellow, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com