Process for folding chemically synthesized polypeptides
A technology for chemical synthesis and polymerization of carriers, which is used in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
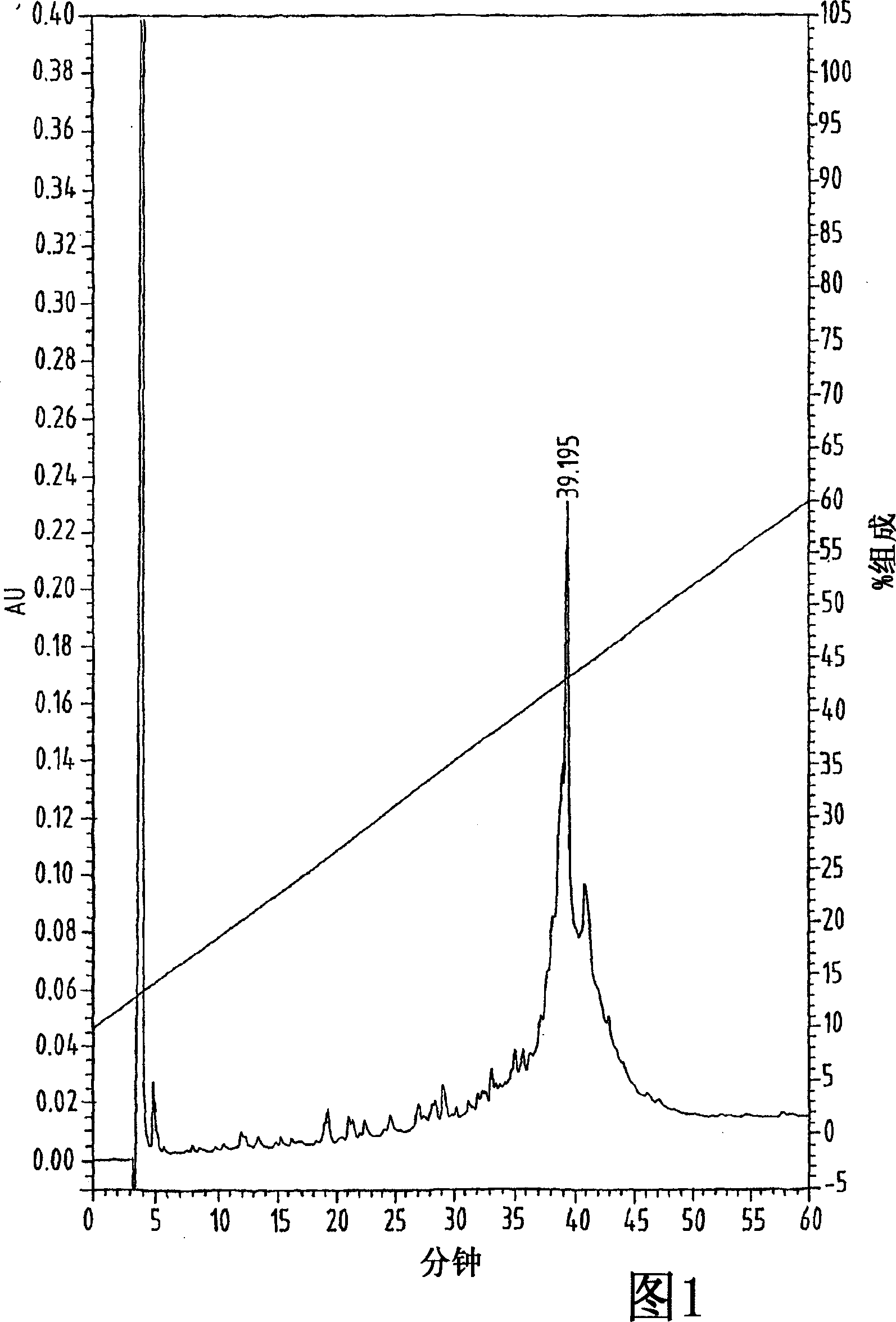
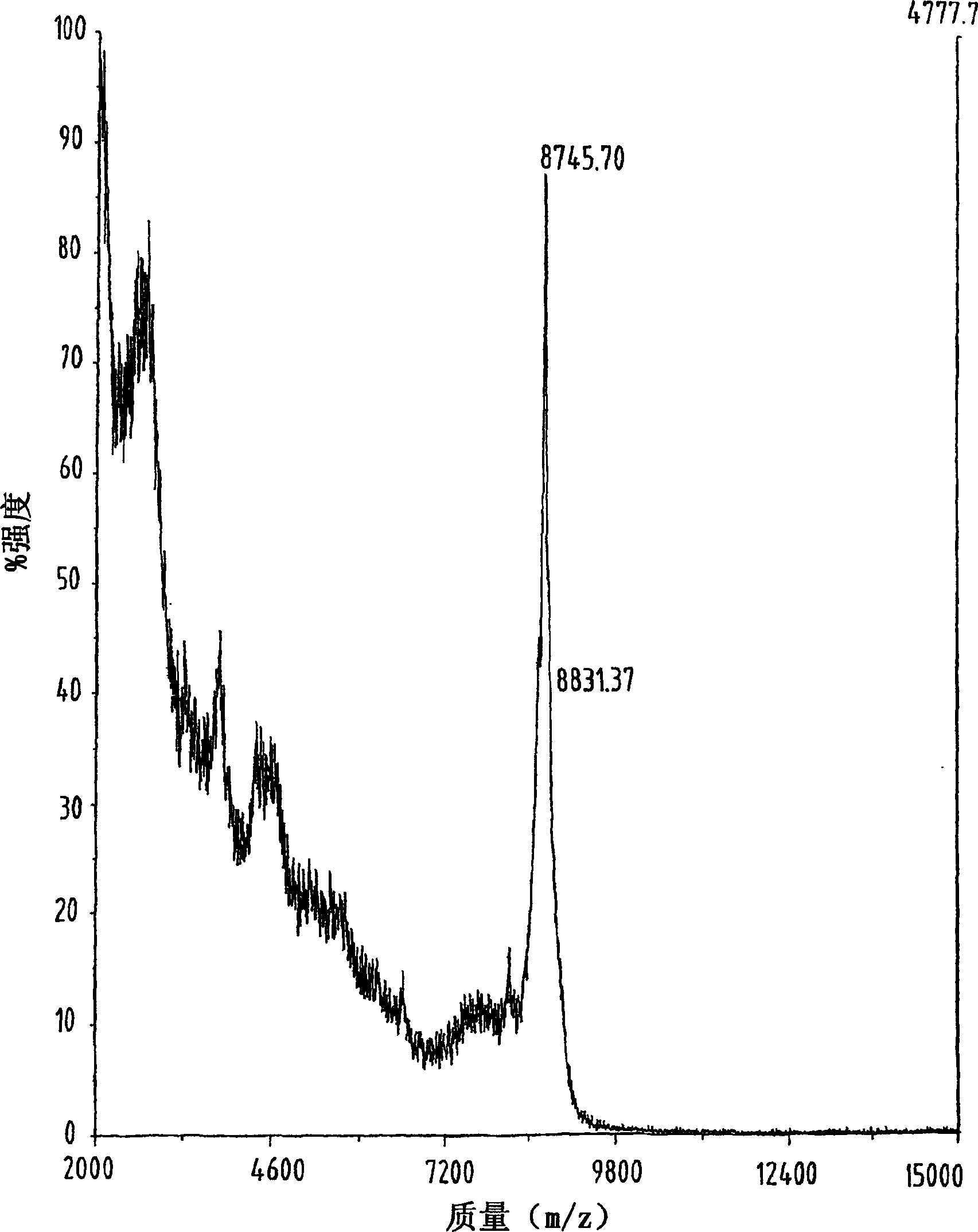
[0079] Example 1 Cys 10,11,34,50 (S-t-Bu)-hu-TARC (thymus and activation-regulated chemokine) synthesis and fold
[0080] Chemokine derivatives with 71 amino acid residues were assembled onto a 433A peptide synthesizer (Perkin Elmer / ABI) using Fmoc / t-Bu chemistry and polystyrene-based resins that were acid-resistant Functionalized with a stable hydroxymethylphenoxyacetic acid linker (Wang resin), Fmoc-Ser(t-Bu) was linked to the on the resin. The degree of substitution is 0.57 mmole / g. The synthesis reaction was carried out on a scale of 0.27 mmole by utilizing a five-fold excess of Fmoc-amino acid and DCI (N, N'-diisopropylcarbodiimide) / HOBt (1-hydroxybenzotriazole) activating reagent in performed in DMF. The coupling reaction time was approximately 60 minutes under conditions where Fmoc deprotection was monitored spectrophotometrically.
[0081] The four cysteine sulfhydryl groups were protected with S-tert-butyl groups and the maximal protection scheme was used...
Embodiment 2
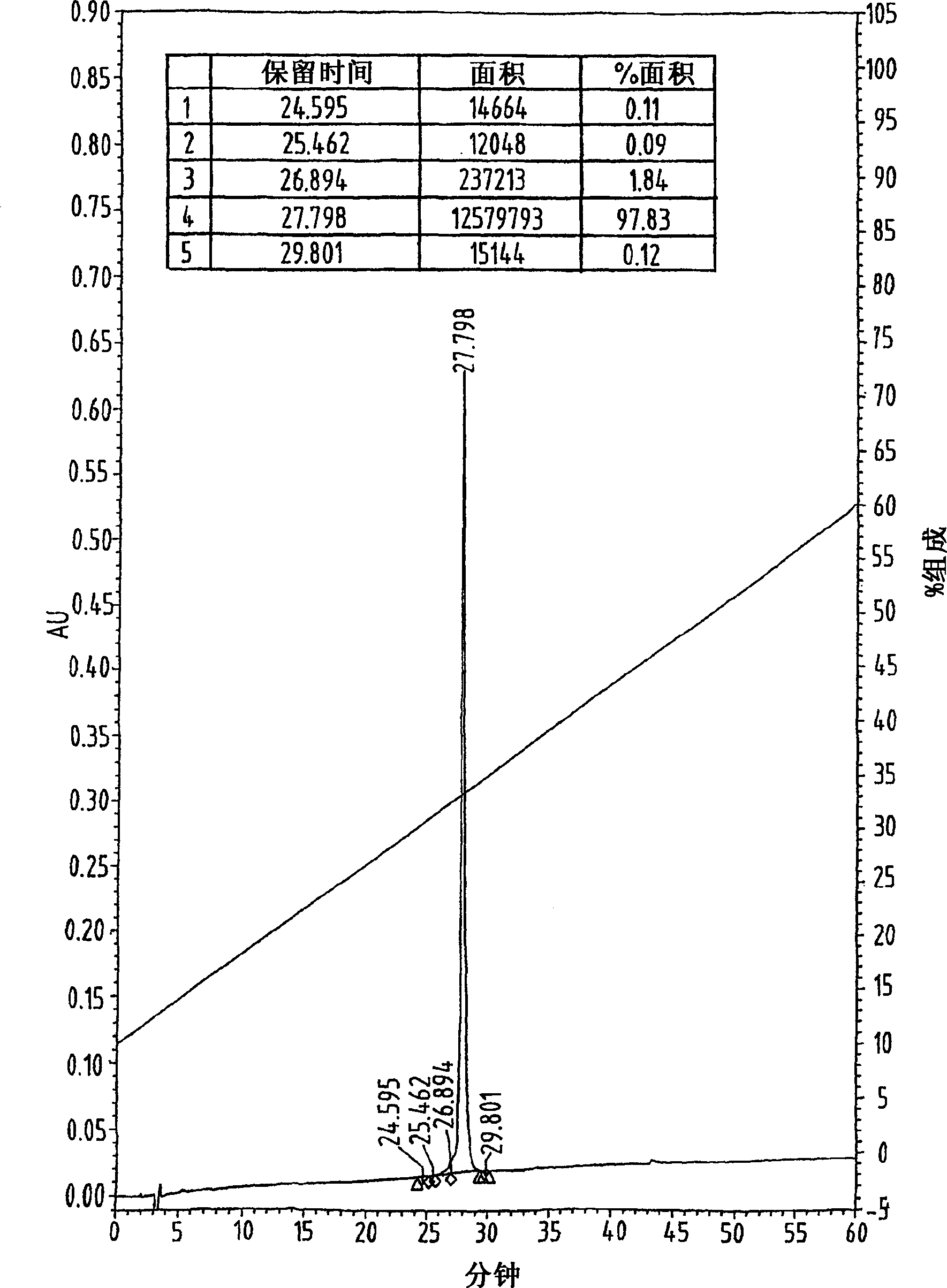
[0089] Example 2 Cys 10,34,50 (S-t-Bu)-hu-TARC and Cys 11,34,50 Synthesis of (S-t-Bu)-hu-TARC and fold
[0090] Cys 10,34,50 (S-t-Bu)hu-TARC and Cys 11,34,50 The conditions used for the synthesis, purification and folding of (S-t-Bu)-hu-TARC derivatives and Cys 10,11,34,50 The conditions adopted by (S-t-Bu)hu-TARC (Example 1) are the same, the only difference is that Cys 10 and Cys 11 Trt protection was performed, and the protecting group was removed at the same time as the cleavage of the polypeptide precursor from the resin. The yields of the final folded chemokines were 80% and 79%, respectively.
Embodiment 3
[0091] Example 3 CVs 34,50 Synthesis and Folding of (S-Bu)-hu-TARC
[0092] In addition to using Trt to Cys 10 and Cys 11 In addition to the protection, the Cys 34,50The conditions adopted for the synthesis, purification and folding of (S-Bu)-hu-TARC derivatives are the same as the conditions adopted for the derivatives of Example 1 and Example 2. is removed. The yield of folded product was about 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com