Prepn of matrine, oxymatrine and sophoxidine from flavescent sophora root
A technology of oxymatrine and production process, applied in the field of matrine, can solve the problems of complicated procedures, high cost, difficult industrialized scale production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
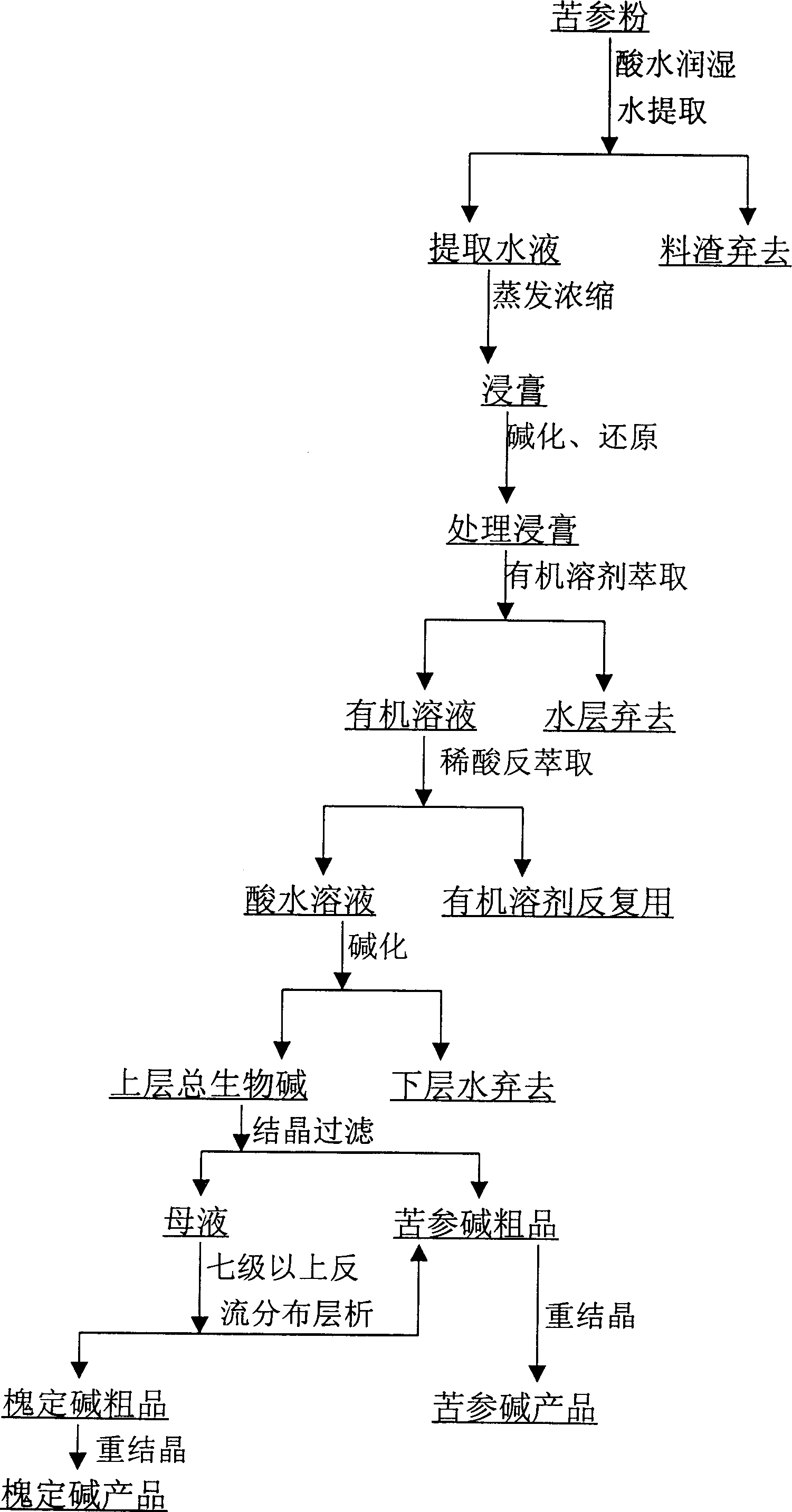
[0014]Select high-quality Sophora flavescens produced in Northwest China, dry it, crush it to 40 meshes, and obtain 200 kg of Sophora flavescens powder, moisten it with 100L of 0.5% H2SO4 solution, leave it for more than 2 hours, put it into a 2000L stainless steel multifunctional extraction tank, and add 800L for the first time Water, heated to 75±5°C, kept at this temperature, forced to circulate water for 2 hours, and filtered out the solution; add 600L water to the multi-functional extraction tank for the second time, heated to 75±5°C, maintained at this temperature, forced to circulate for 2 hours, filtered out of the solution; add 500L of water to the multifunctional extraction tank for the third time, heat at 75±5°C, keep the temperature, force the water to circulate for 2 hours, and filter out the solution; combine the three extracts, filter out the clear liquid, and use double-effect evaporation Concentrate at 80°C and under vacuum conditions below -0.08MPa, evaporate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com