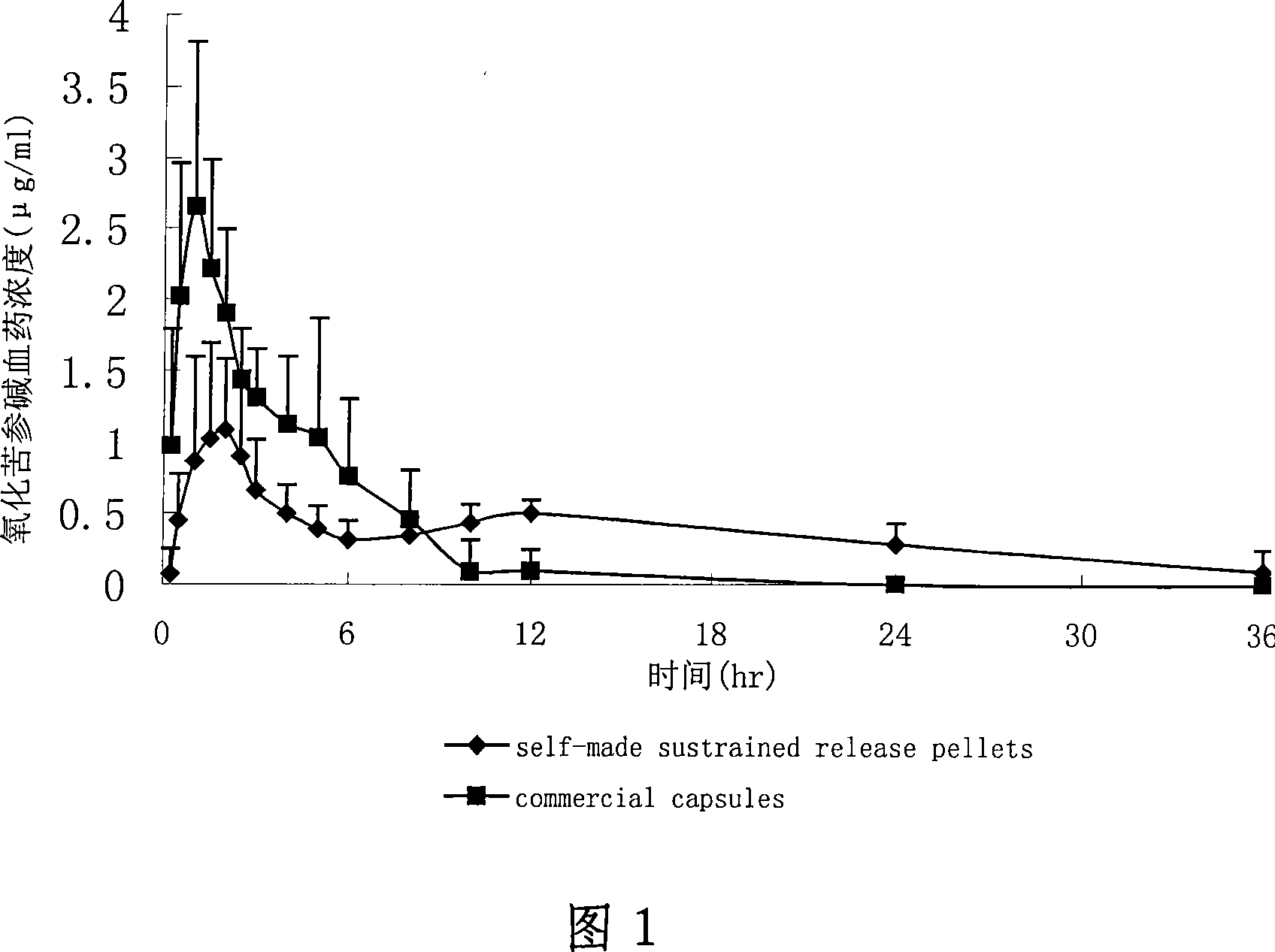
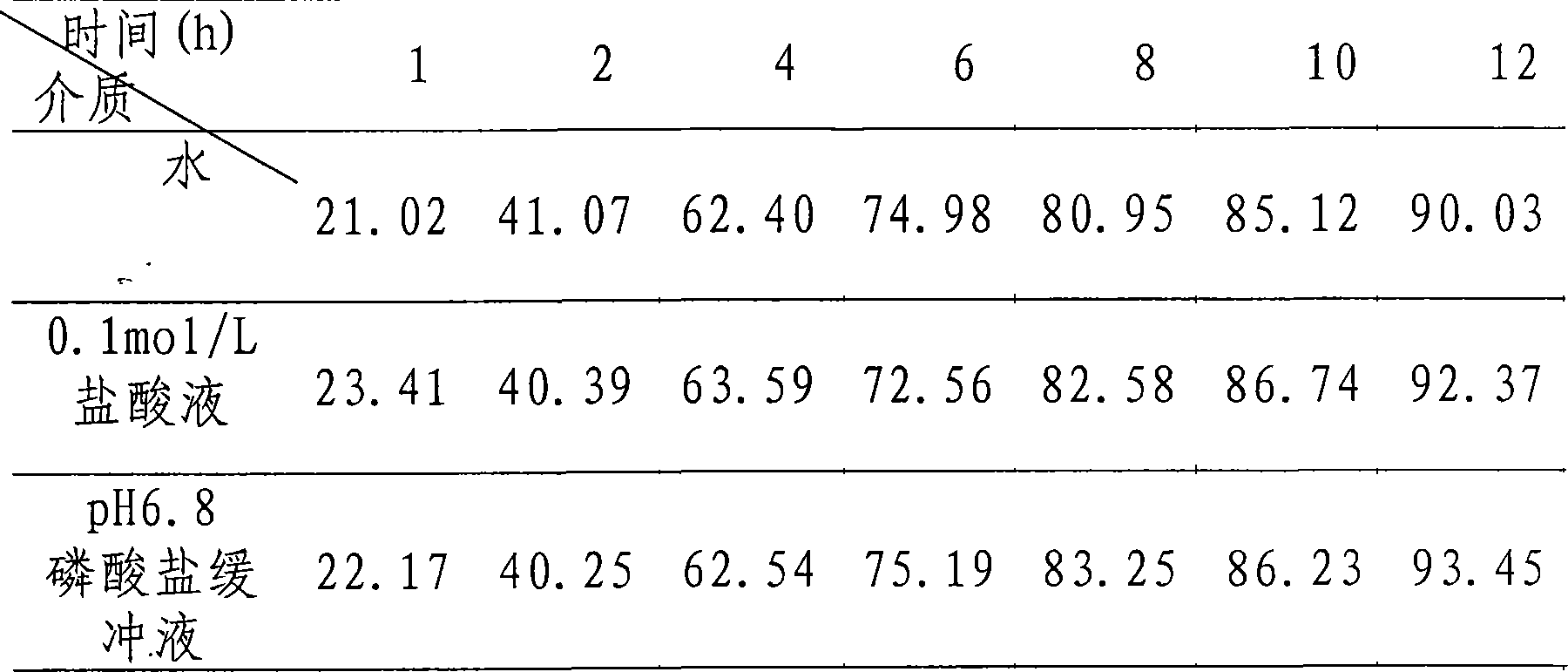
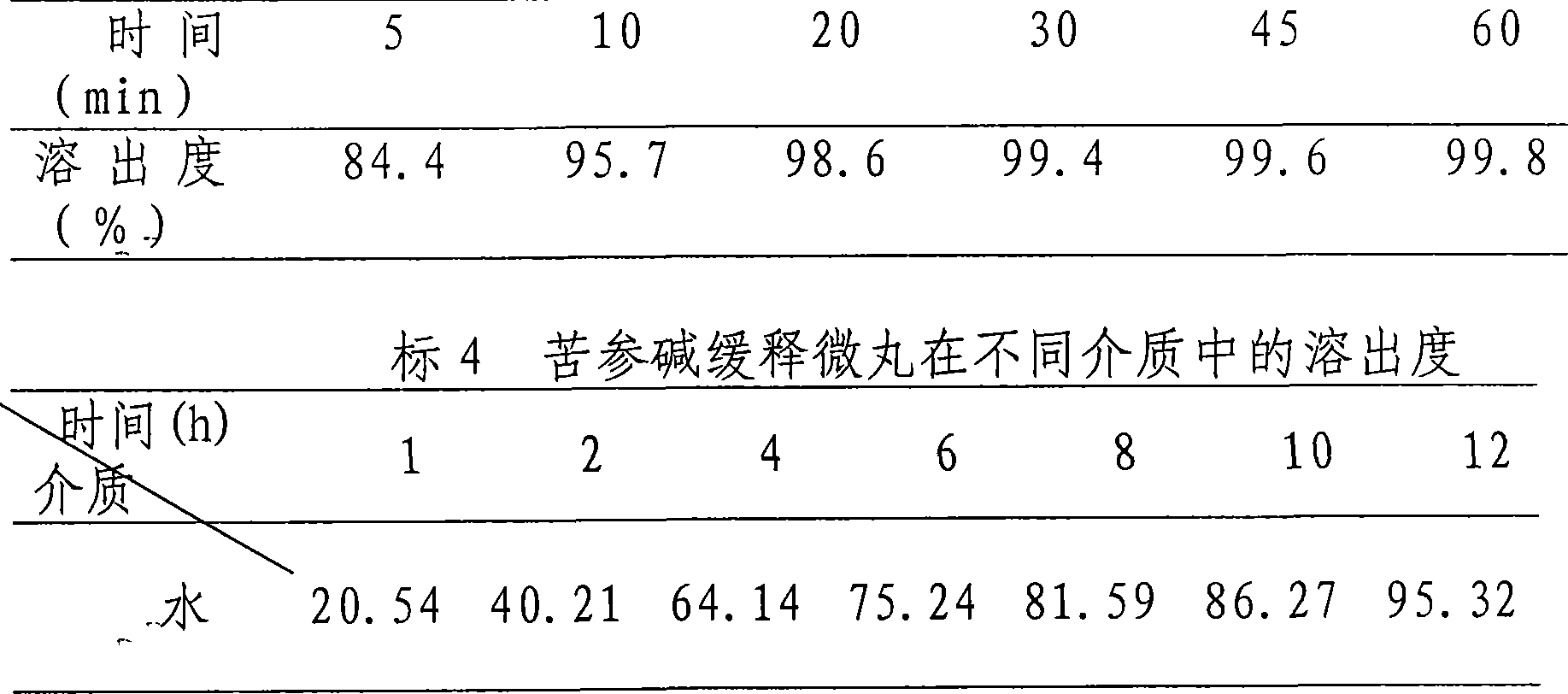
Oxymatrine or matrine sustained-release pellet and preparation method thereof
A technology of slow-release pellets and matrine, applied in the field of medicine, can solve the problems of not being suitable for clinical needs, not easy to control the release rate, and large differences between batches of products, so as to prolong maintenance and reduce side effects Response, the effect of reducing the peak and valley phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Preparation of binder Magnetically stir 24 grams of hydroxypropyl methylcellulose in 800 ml of distilled water at room temperature for 2-8 hours, and filter through a 100-mesh sieve after completely dissolving.
[0056] (2) Preparation of slow-release coating solution Add 0.2 g of sodium lauryl sulfate and 9.24 g of talc powder into 100 mL of water, stir for 2 hours to make it uniform, and slowly pour the above suspension into 70 g of Eudragit NE 30D , add water to 250mL, stir well. Filter through 80 mesh sieve. Stir continuously during the coating process.
[0057] (3) Preparation of drug-containing layer 300 grams of dry blank pellet cores were sprinkled with 300 grams of matrine evenly and slowly under the atomized adhesive, and dried at room temperature.
[0058] (4) Preparation of the sealing layer Continue spray coating with 50ml of adhesive, and dry at room temperature.
[0059] (5) Preparation of sustained-release coating layer Take 300 grams of 24-20 mes...
Embodiment 2
[0061] (1) Preparation of the adhesive, using water as the adhesive
[0062] (2) Preparation of slow-release coating solution Add 0.21 gram of sodium lauryl sulfate, 9.95 gram of talcum powder, and 4.2 gram of triethyl citrate into 100 mL of water, stir for 2 hours, make uniform, and slowly dissolve the above suspension Pour into 50 grams of Eudragit RS30D, add water to 250mL and stir well. Filter through 80 mesh. Stir continuously during the coating process.
[0063] (3) Preparation of drug-containing layer 400 grams of dry blank pellet cores were sprinkled with 280 grams of matrine evenly and slowly under the atomized adhesive, and dried at room temperature.
[0064] (4) Preparation of the sealing layer Use 50 ml of 3% hydroxypropyl methylcellulose aqueous solution to continue spray coating, and dry at room temperature.
[0065] (5) Preparation of slow-release coating layer Take 300 grams of pill cores with 28-24 mesh, continue to spray coating with slow-release coating s...
Embodiment 3
[0067] (1) Preparation of binder Magnetically stir 16 g of hydroxypropyl methylcellulose in 800 ml of distilled water at room temperature for 2-8 hours, and filter through a 100-mesh sieve after completely dissolving.
[0068] (2) Preparation of sustained-release coating solution Dissolve 1.35 g of polyethylene glycol 6000 in 100 mL of water (heating to aid dissolution), add 0.22 g of sodium lauryl sulfate, and 10.5 g of talc, and stir for 2 hours to make it uniform. Slowly pour the above suspension into 60 grams of Eudragit RS 30D and Eudragit RL30D (10:1), add water to 250 mL and stir well. Filter through 80 mesh. Stir continuously during the coating process.
[0069] (3) Preparation of drug-containing layer 400 grams of dry blank pellet cores were sprinkled with 280 grams of matrine evenly and slowly under the atomized adhesive, and dried at room temperature.
[0070] (4) Preparation of the sealing layer Continue spray coating with 50ml of adhesive, and dry at room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com