Process for producing fluoroalkanol
A technology of fluorine-containing alkanols and manufacturing methods, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., and can solve problems such as difficulty in controlling the addition number of tetrafluoroethylene, low concentration, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Manufacture of 2,2,3,3-tetrafluoro-1-propanol
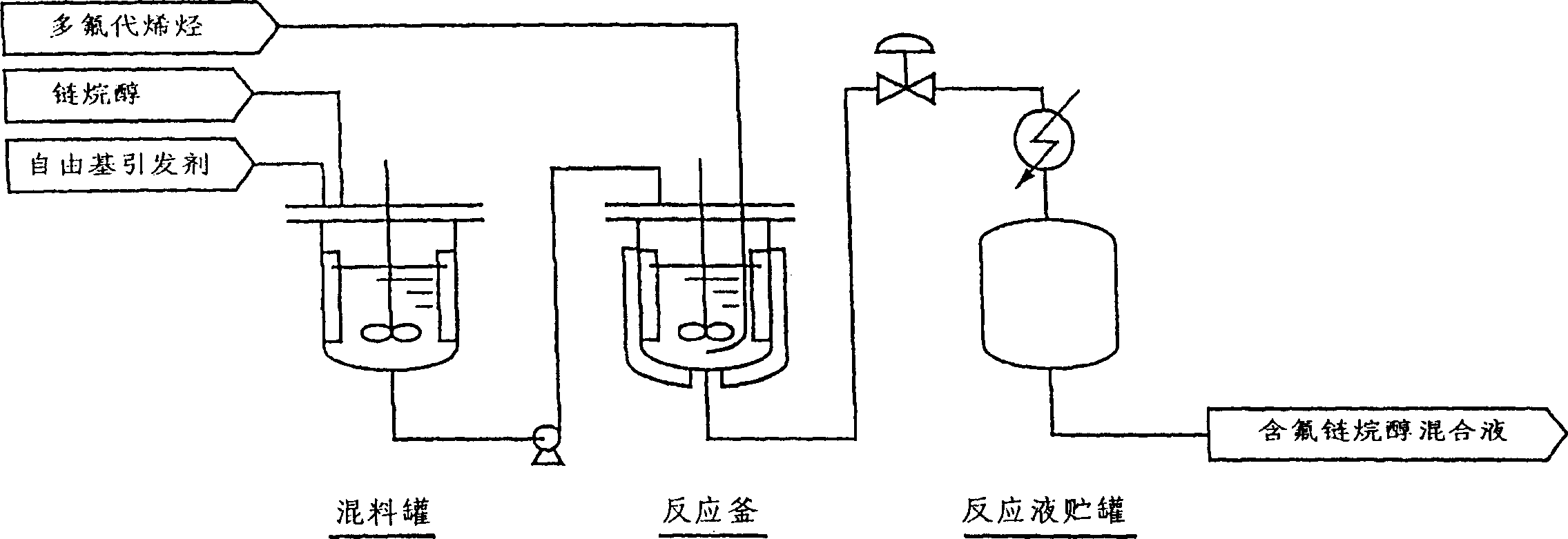
[0055] according to figure 1 The reaction was carried out as described in the flowchart. That is to say, with a 1-meter 3 A reaction kettle made of corrosion-resistant nickel-based alloy (Hastelloy) was used as a reactor, and 341 kg (432 L) of methanol was added therein, and the inner temperature was heated to 125°C. While maintaining this temperature, supply tetrafluoroethylene to the reactor so that the pressure is 0.9 MPa. In the initial stage of the reaction, use a metering pump to mix 5.5 kilograms of di-tert-butyl peroxide and 44 kilograms of methanol in the mixing tank. The mixed solution was continuously supplied at 25 L / hour for 0.5 hours, and then continuously supplied at 4.2 L / hour for 9 hours to perform a reaction.
[0056] Then, while continuously supplying tetrafluoroethylene so that the pressure in the reactor is 0.9 MPa, the solution mixed in the mixing tank at a ratio of 12 kg of di-tert-butyl peroxide ...
Embodiment 2
[0059] Manufacture of 2,2,3,4,4,4-perfluoro-1-butanol
[0060] according to figure 1 The reaction was carried out as described in the flowchart. That is to say, with a 1-meter 3 A reaction kettle made of corrosion-resistant nickel-based alloy (Hastelloy) was used as a reactor, and 170 kg (212 L) of methanol was added therein, and the inner temperature was heated to 130° C. In the initial stage of reaction, 2.8 kilograms of di-tert-butyl peroxide and 22 kilograms of methanol in the mixing tank were mixed with a metering pump to form a solution that was continuously supplied to the reactor for 0.25 hours at 25L / hour, and then continuously supplied at 4.2L / hour for 4 hours. react. At the same time, 20 kg of hexafluoropropane was continuously supplied to the reactor at a rate of 3 L / hour by a metering pump. Then, use a metering pump to mix 12 kg of di-tert-butyl peroxide with 315 kg of methanol to form a solution that is continuously supplied to the reactor at 28 L / hour, and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com