Sodium borohydride catalytic hydrolysis process and reactor of generating hydrogen
A technology of sodium borohydride and catalytic hydrolysis, applied in the production of hydrogen and other directions, can solve the problem of low catalytic activity, and achieve the effects of high hydrogen purity, safe use, and no deactivation problem.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
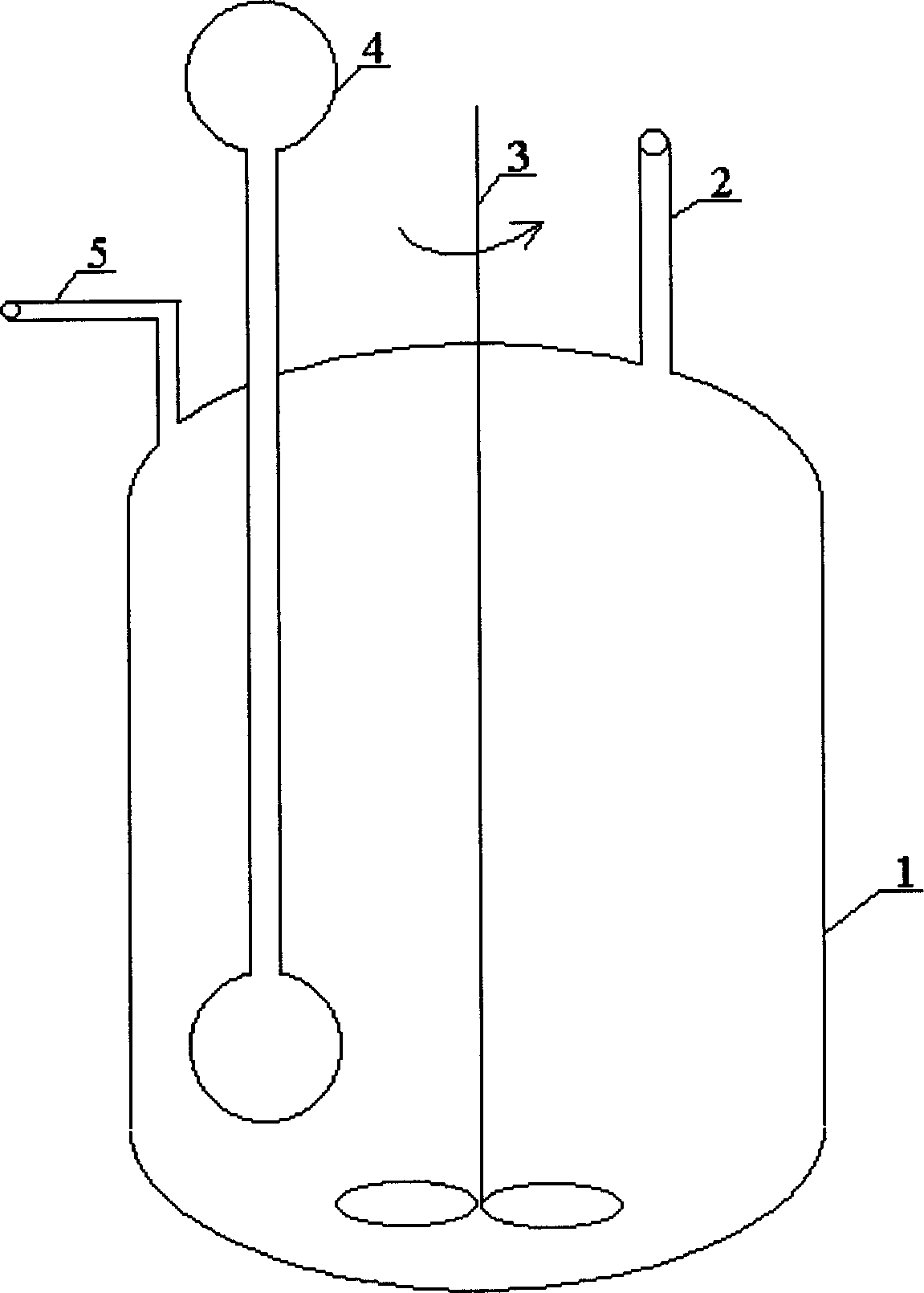
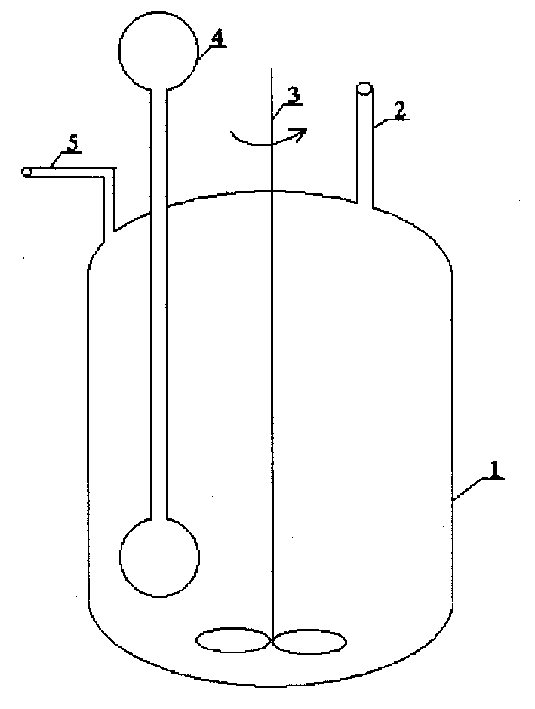
[0019] In a 500ml reactor with a mechanical stirrer, a dumbbell-shaped double-chamber connected tube vapor-liquid circulation heat exchanger with a 50ml evaporation chamber and a 100ml condensation chamber is installed. 30ml of ethanol is housed in the double-chamber connected tube vapor-liquid circulation heat exchanger. Add 1.6g CoCl to the reactor 2 .6H 2 O, 0.92gFeCl 3 .6H 2 O and 20ml of water. At a stirring speed of 500 rev / min, continuously add an aqueous solution containing 20% (mass) sodium borohydride and 10% (mass) sodium hydroxide with a liquid feed pump at a speed of 3 g / min, and measure with a mass flow indicator The rate at which hydrogen gas is produced. A total of 90 g of an aqueous solution containing 20% (mass) sodium borohydride and 10% (mass) sodium hydroxide was added within 30 minutes. The hydrolysis is complete immediately after the sodium borohydride solution is added to the reactor. The average rate of hydrogen generation is 1.4 liters / minu...
example 2
[0021] In a 500ml reactor with a mechanical stirrer, a dumbbell-shaped double-chamber connected tube vapor-liquid circulation heat exchanger with a 50ml evaporation chamber and a 100ml condensation chamber is installed. 30ml of ethanol is housed in the double-chamber connected tube vapor-liquid circulation heat exchanger. Add 1.6g CoCl to the reactor 2 .6H 2 O and 20ml of water. At a stirring speed of 500 rev / min, continuously add an aqueous solution containing 20% (mass) sodium borohydride and 10% (mass) sodium hydroxide with a liquid feed pump at a speed of 3 g / min, and measure with a mass flow indicator The rate at which hydrogen gas is produced. A total of 90 g of an aqueous solution containing 20% (mass) sodium borohydride and 10% (mass) sodium hydroxide was added within 30 minutes. After the addition of the sodium borohydride solution was complete, stirring was continued. Sodium borohydride hydrolysis was complete after 62 minutes. The average rate of hydrogen ...
example 3
[0023] In a 500ml reactor with a mechanical stirrer, a dumbbell-shaped double-chamber connected tube vapor-liquid circulation heat exchanger with a 50ml evaporation chamber and a 100ml condensation chamber is installed. 30ml of ethanol is housed in the double-chamber connected tube vapor-liquid circulation heat exchanger. Add 2.72g FeCl to the reactor 3 .6H 2 O and 20ml of water. At a stirring speed of 500 rev / min, continuously add an aqueous solution containing 20% (mass) sodium borohydride and 10% (mass) sodium hydroxide with a liquid feed pump at a speed of 3 g / min, and measure with a mass flow indicator The rate at which hydrogen gas is produced. A total of 90 g of an aqueous solution containing 20% (mass) sodium borohydride and 10% (mass) sodium hydroxide was added within 30 minutes. After the addition of the sodium borohydride solution was complete, stirring was continued. Sodium borohydride hydrolysis was complete after 150 minutes. The average rate of hydroge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com