Modified blood coagulation factor VIII
A technology of coagulation factor and human coagulation factor, which is applied in the direction of coagulation/fibrinolytic factor, VII factor, blood diseases, etc., can solve the problems of insufficient supply, difficult separation and purification, immunogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] Preparation of Recombinant Coagulation Factor VIII
[0088] The eukaryotic protein expression system can be used to prepare recombinant coagulation factor VIII. Generally, a vector containing the defective gene and the recombinant DNA to be expressed is used to transform eukaryotic cell lines with defective genes. Transformation can be achieved by techniques such as electroporation or virus delivery. The cell line used to produce the protein should be compatible with the protein of interest, be able to continuously express the protein of interest, and be able to grow on a medium that is conducive to the purification of the protein of interest, as well as factors known to those skilled in the art. Examples of these techniques are disclosed in European Patent Application 0 302 968 A2 and US Patent No. 5,149,637, both of which are incorporated herein by reference.
[0089] Assay of Recombinant Coagulation Factor VIII
[0090] In at least two types of clinical trials, the recom...
Embodiment 1
[0114] Example 1: Construction of fVIII mutant cDNA
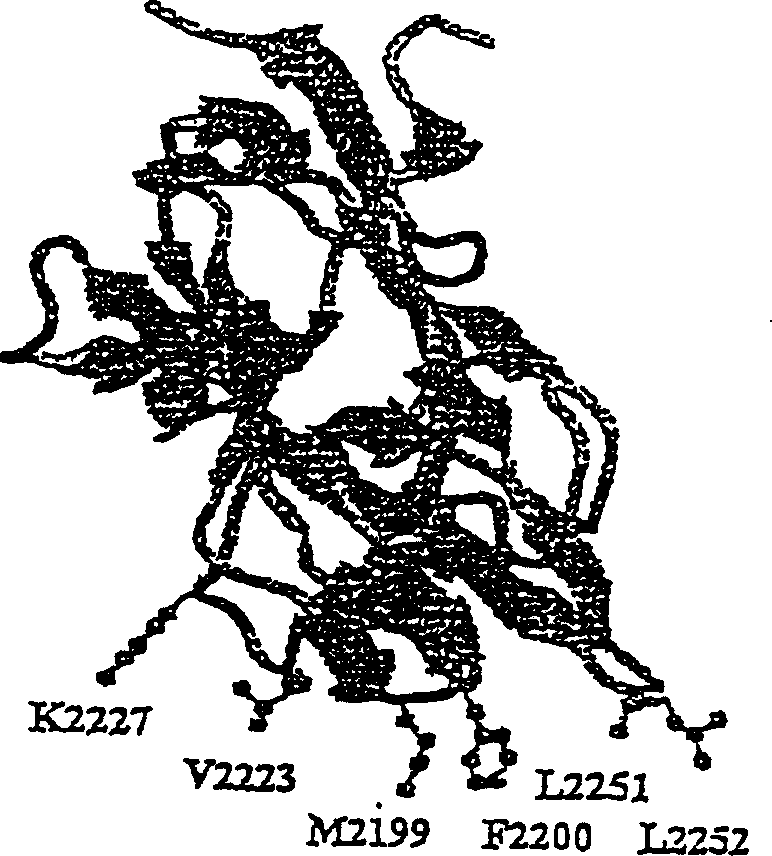
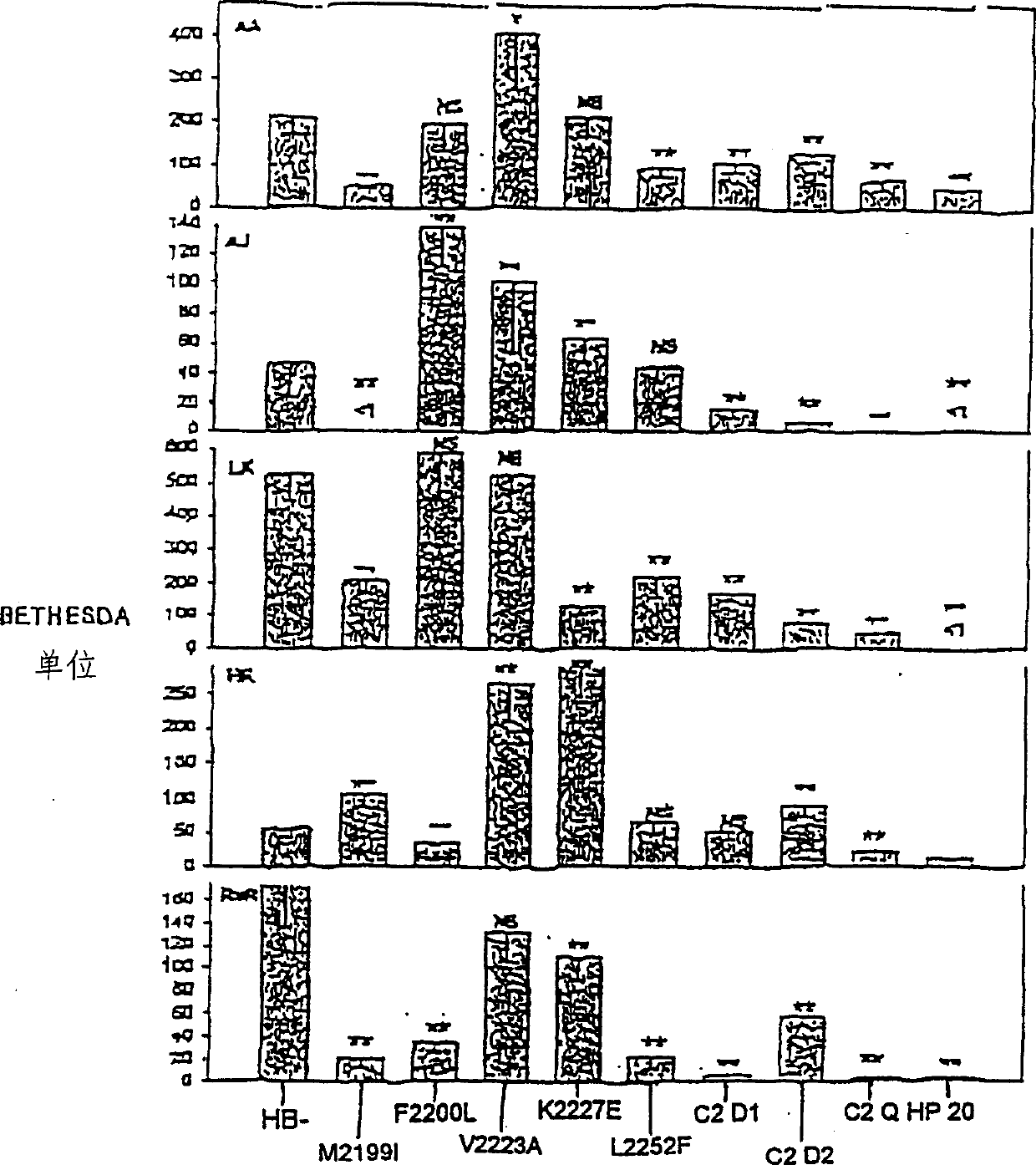
[0115] Through SOE mutagenesis, mutations are made in HSQ codons to produce the following proteins: Met2199Ile (human to pig), ATG to ATC; Phe2200Leu (human to dog), TTT to TTG; Val2223Ala (human to dog), GTG to GCC; Lys2227Glu (human to pig), AAA to GAG; Leu2252Phe (human to mouse), CTT to TTC; Met2199Ile / Phe2200Leu, ATG to ATC and TTT to TTG; Val2223Ala / Lys2227Glu, GTG to GCC and AAA to GAG; Met2199Ile / Phe2200Leu / Val2223Ala / Lys2227Glu, ATG to ATC, TTT to TTT, GTG to GCC, and AAA to GAG.
[0116] HSQ / ReNeo is used as a template in the PCR reaction. The first PCR reaction uses human C1 primers, SEQ ID NO: 3, 5'-GTG GAT TCA TCT GGG ATA AAA CAC-3', called H3763+, corresponding to nucleotides 3763-3786 in the HSQ sequence, as the sense Primer. The following primers are used as antisense primers: Met2199Ile, SEQ ID NO: 4, 5'-AGG AGA CCA GGT GGC AAA GAT ATT GGT AAA GTA GGATGA-3', Phe2200Leu, SEQ ID NO: 5, 5'-TGA AGG AGA CCA GGT GGC...
Embodiment 2
[0120] Example 2: Expression of recombinant fVIII molecule
[0121] The transfected cell line was maintained in Dulbecco's modified Eagles medium F12 containing 10% fetal bovine serum, 50 U / ml penicillin and 50 μg / ml streptomycin. Fetal bovine serum was heat-inactivated at 56°C for 1 hour before use. The mutant cDNA in ReNeo was stably transfected into BHK cells, the geneticin resistance was screened, and it was changed to serum-free, AIM-V medium for expression, and heparin-agarose chromatography was used as described above (Healey, JF Et al., supra, 1998) partial purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com