Plasmid for gene therapy of cardiac muscle
A gene therapy and plasmid technology, applied in gene therapy, genetic material components, cardiovascular system diseases, etc., can solve the problems of reducing the specificity and effectiveness of gene therapy, and cannot control the specific expression of target genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
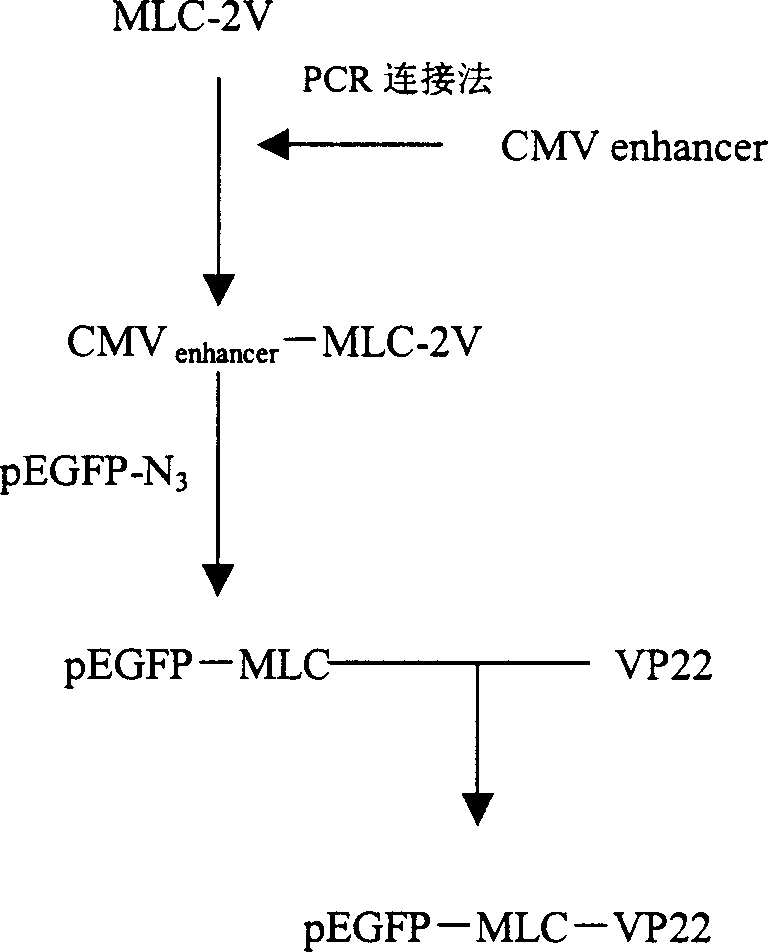
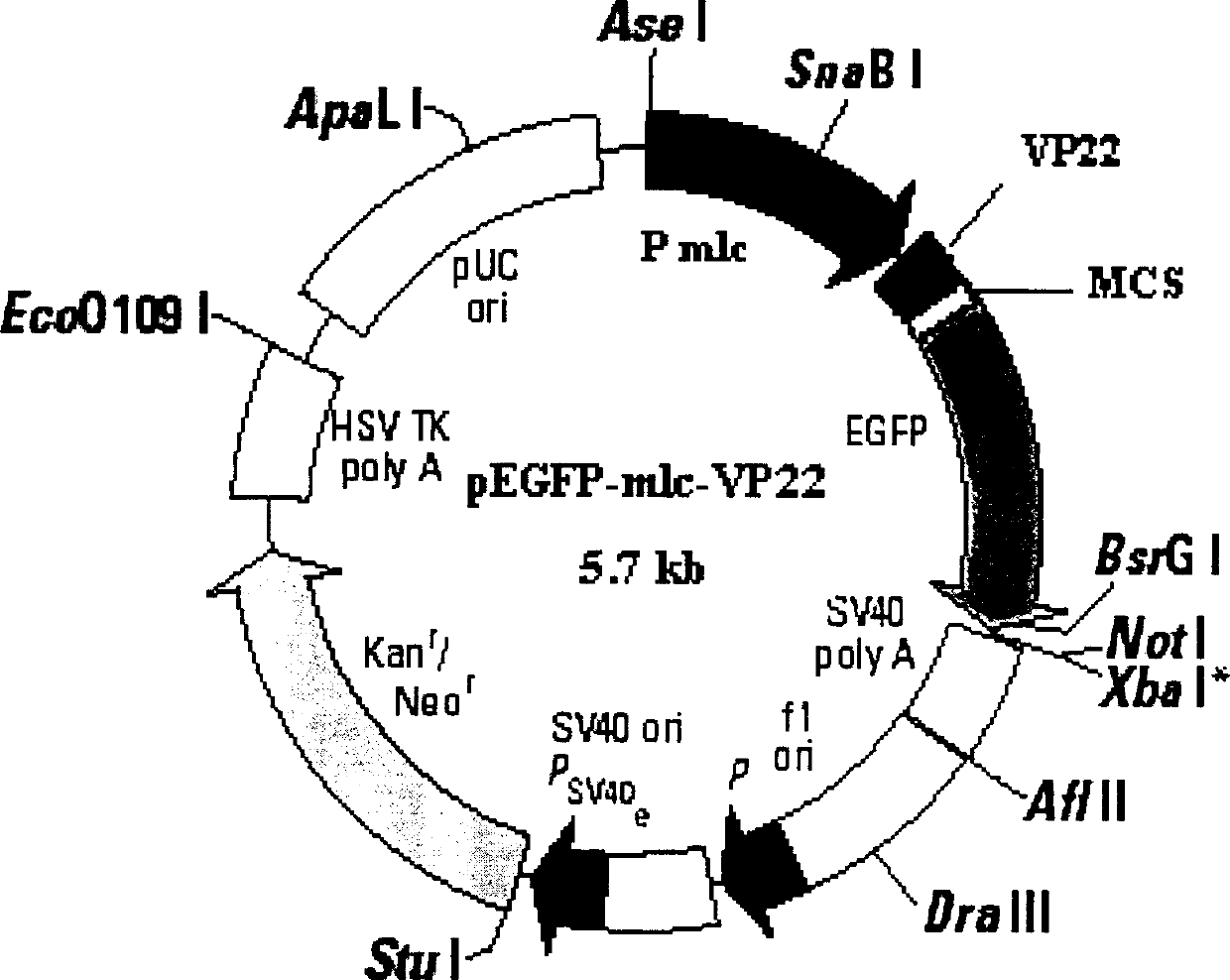
[0027] Example 1 Construction of pEGFP-MLC-VP22 plasmid
[0028] The CMV enhancer-MLC-2V junction fragment was prepared by PCR amplification ligation method, and the original pEGFP-N fragment was replaced by directional cloning method 3 The CMV increaser and promoter of the vector were made into pEGFP-MLC vector; the VP22 gene with Bgl II and HindIII recognition sequences were introduced into the 5' and 3' ends respectively, and inserted into the pEGFP-MLC vector downstream of the MLC-2V promoter by directional cloning The pEGFP-MLC-VP22 vector was constructed between the Bgl II and HindIII sites.
[0029] The specific method is as follows:
[0030] According to the known human MLC-2V promoter sequence, design synthetic PCR primers: upstream primer, 5'-AATGGGAGTTTGTTTTGGACCCAGAGCACAGAG-3', downstream primer, 5'-TAATA GCTAGC GGCCGGCCCCTGCTGT-3'(Nhe I), using human genomic DNA as a template, was amplified to obtain the MLC-2V fragment (250 bp). The sequence SEQ ID NO: 2 is a...
Embodiment 2
[0059] Example 2 Plasmid amplification, extraction and purification
[0060] Using protozoa Escherichia coli as the host cell, pEGFP-MLC-VP22 was amplified, extracted and purified by conventional molecular biology methods. For specific operations, see the literature "Molecular Cloning Experiment Guide" ([US] J. Sambrook, E.F. Fritsch, T. Maniartis, second edition).
Embodiment 3
[0061] Example 3 Cytology experiment
[0062] The same amount of pEGFP-N was transferred by liposome method 3 , pEGFP-MLC and pEGFP-MLC-VP22 were transfected with cultured human Hela (human uterine cancer cell line), Ea.hy926 (human embryonic umbilical vein endothelial cells), IMA (human inferior mesenteric artery media smooth muscle cell line) and Hep2 (human liver cancer cell line), after 24 hours of transfection, observe the expression of GFP in the cells of each group. It was found that: pEGFP-N 3 GFP can be expressed in the above cells, but neither pEGFP-MLC nor pEGFP-MLC-VP22 expresses GFP.
[0063] The same amount of pEGFP-N was transferred by liposome method 3 , pEGFP-MLC and pEGFP-MLC-VP22 were transfected into isolated and cultured neonatal mouse cardiomyocytes, and after 24 hours of transfection, the expression of GFP in the cells of each group was observed. It was found that: pEGFP-N 3 GFP was not expressed, but both pEGFP-MLC and pEGFP-MLC-VP22 had GFP expres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com