Quinoline derivative and quinazoline derivative inhibiting self-phosphorylation of hepatocytus proliferator receptor and medicinal composition containing the same
A kind of technology of compound and solvate, applied in the field of quinoline derivatives and quinazoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0464] Compound preparation
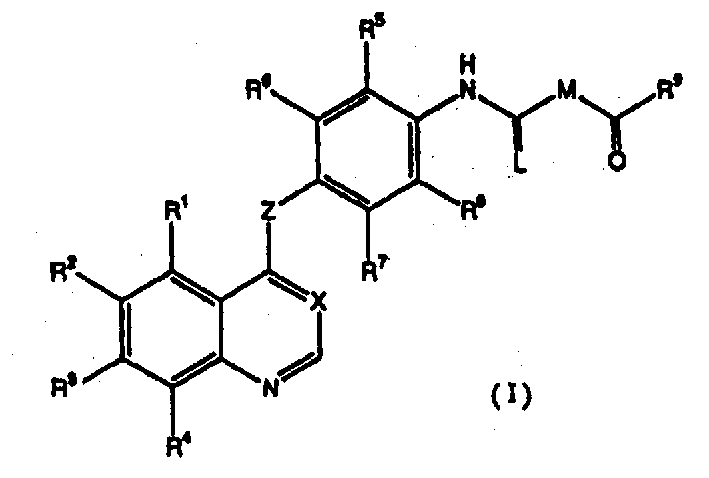
[0465] Compounds of the invention can be prepared, for example, according to Schemes 1-9. The raw materials necessary for the synthesis of the compounds of the present invention are commercially available or can be easily prepared by conventional methods. Program R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 18 , R 19 and X are the same as defined above. PG stands for protecting group, R 3’ O represents an alkoxy group that may be substituted, Hal represents a halogen atom, R 51 and R 52 Can be the same or different, denote C that can be substituted 1-6 Alkyl, or R 51 and R 52 A saturated or unsaturated 3-8 membered heterocyclic ring can be formed together with the nitrogen atom connected to it, and n represents an integer of 1-6.
[0466] Scheme 1: Preparation of 4-(aminophenoxy) quinoline derivatives and corresponding quinazoline derivatives prepare
[0467]
[0468] 4-Chloroquinoline derivat...
Embodiment
[0515] The invention is illustrated by the following examples, but the invention is not limited to these examples.
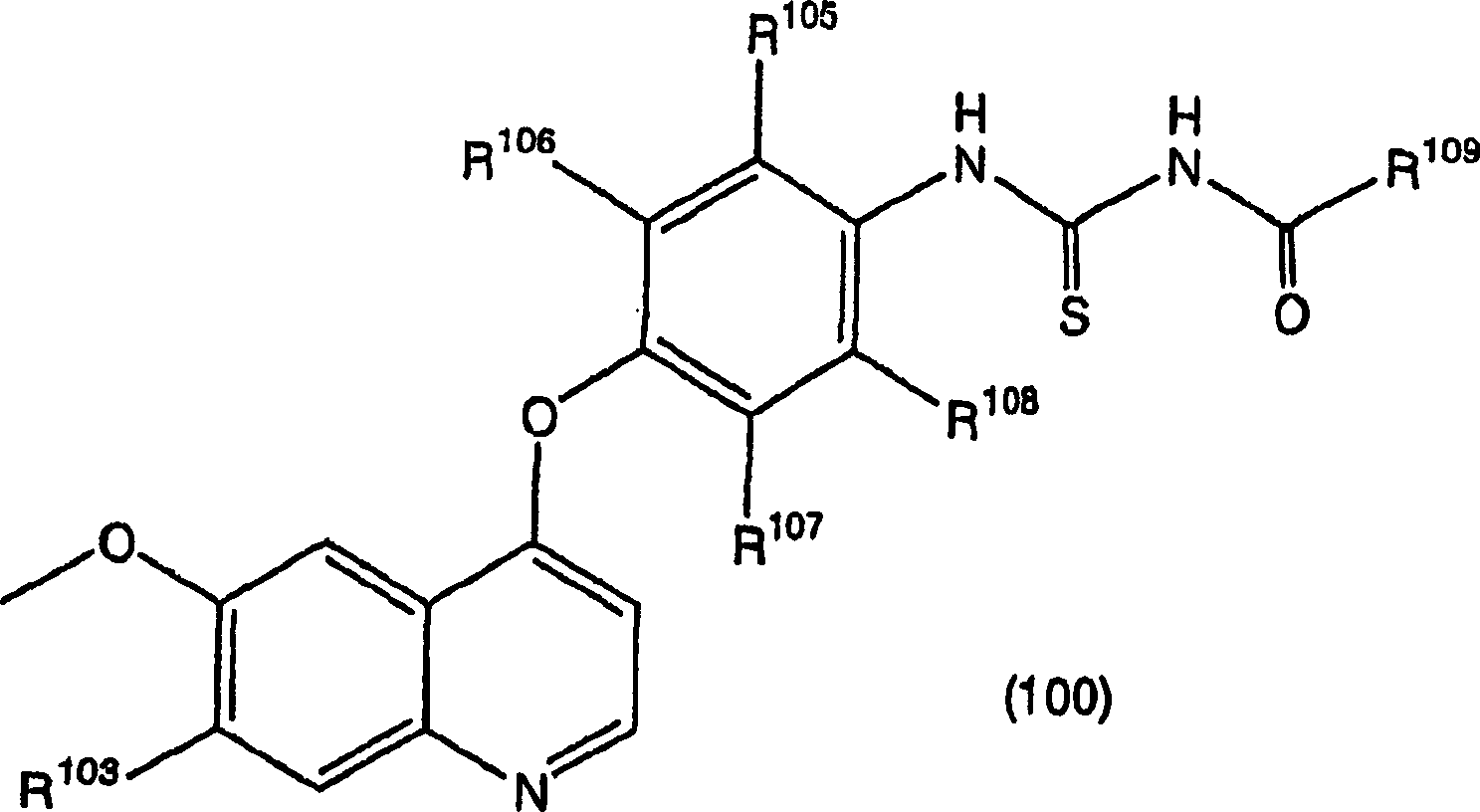
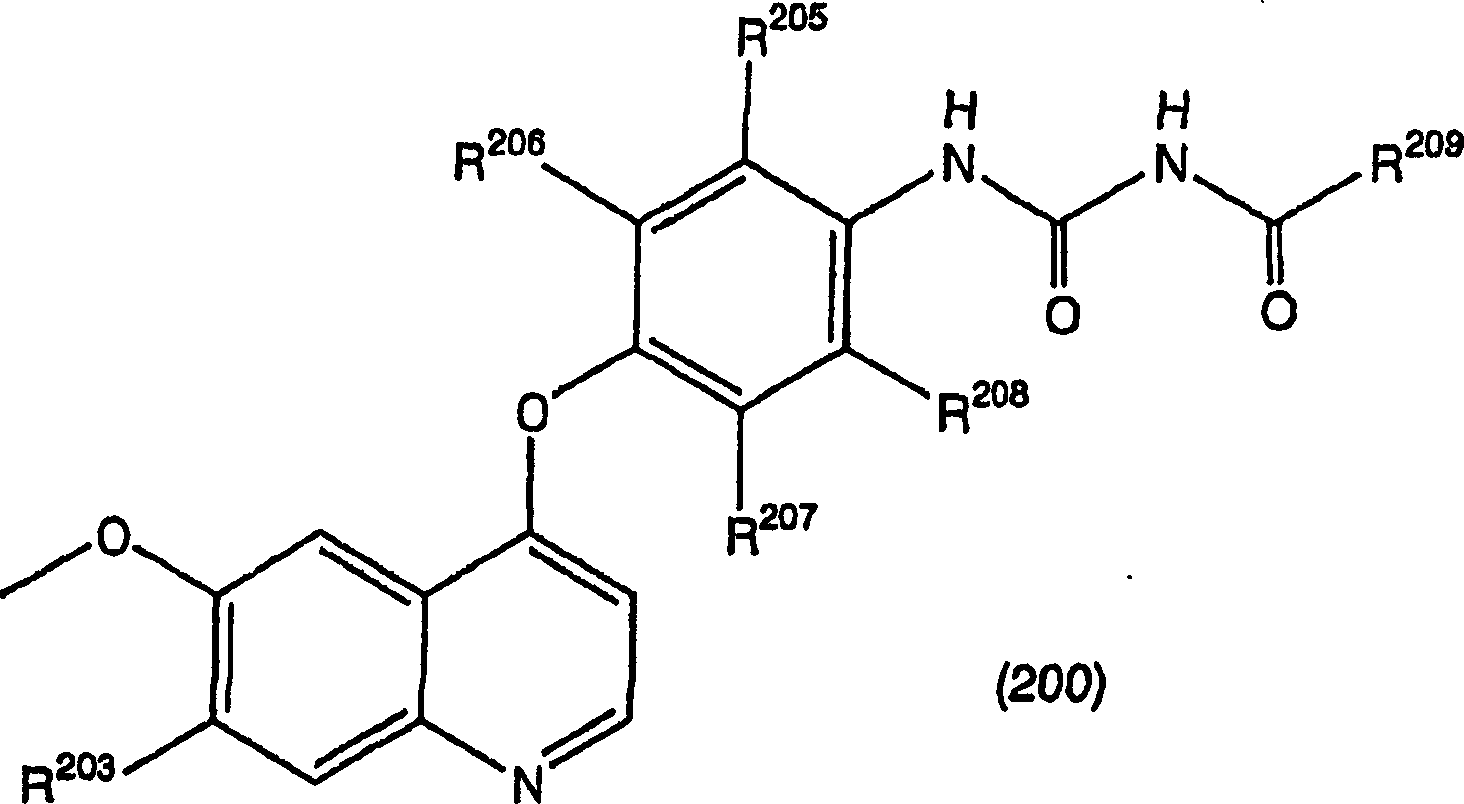
[0516] The raw materials required for the synthesis can be prepared according to the records of WO97 / 17329, WO98 / 47873, WO00 / 43366 and JP-A-9-328782. Raw materials not described in these publications were prepared as shown in the following Preparation Examples.
[0517] Scheme 10: Preparation of starting materials 1-10
[0518]
[0519] Scheme 11: Preparation of starting materials 11 and 12
[0520]
preparation example 1
[0521] Preparation example 1 (raw material 1)
[0522] Add 7-(benzyloxy)-4-chloro-6-methoxyquinoline (29g), 3-fluoro-4-nitrophenol (20g), N,N-diisopropylethylamine (33ml) , Chlorobenzene (14ml), heated and stirred at 140°C for 15 hours. After completion of the reaction, 2N aqueous sodium hydroxide solution (30 ml) was added, followed by stirring at room temperature for 3 hours. Water was added to the reaction liquid, extracted with chloroform, and the chloroform layer was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 40 g of the target compound in a yield of 50%.
[0523] 1 H-NMR (CDCl 3 , 400MHz): δ8.58(d, J=5.1Hz, 1H), 8.48-8.44(m, 1H), 8.21-8.19(m, 1H), 7.64-7.35(m, 8H), 6.79(d, J =5.1Hz, 1H), 5.33(s, 2H), 3.94(s, 3H)
[0524] Mass spectral analysis value (m / z): 421[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com