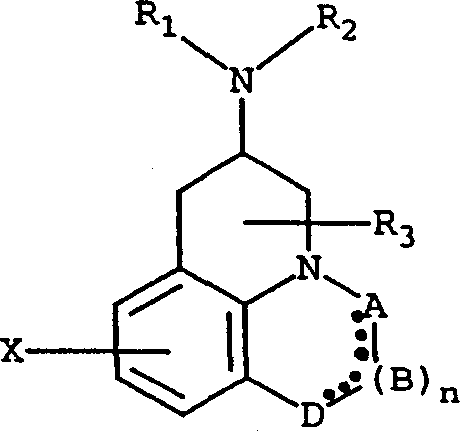
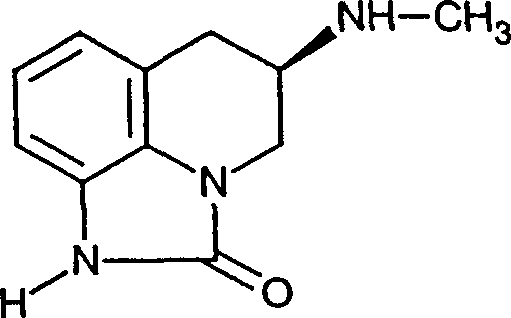
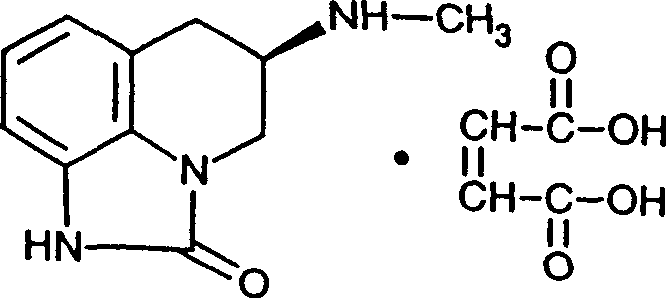
Compounds for treating fibromyalgia and chronic fatigue syndrome
A technology for chronic fatigue and fibromyalgia, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve problems such as damage, no permanent elimination, addiction to the liver, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] 1-Benzyl-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one (II)
[0116] 4H-imidazo[4,5,1-ij]quinolin-2(1H)-one (I, J.Heterocyclic Chem., 19,837-49(1982) 1.0g, 5.8 mmol) and DMF (10ml) was cooled to 0°C and then treated with a mixture of potassium tert-butoxide and THF (1.98M, 3.2ml, 6.3mmol) keeping the reaction temperature at 0°C. The resulting mixture was stirred at 0°C for 10 minutes. Then benzyl bromide (0.73ml, 6.1mmol) was added while maintaining the reaction temperature, extracted from water with methyl tert-butyl ether (MTBE), and washed several times with water. The MTBE phase was concentrated under reduced pressure. The concentrate was cooled to 0°C, filtered and rinsed twice with 0°C MTBE. The product was dried by blowing nitrogen gas under reduced pressure at 50° C. to obtain the title compound, CMR (CDCl 3 , 100MHz) 153.78, 136.44, 128.69, 127.67, 127.60, 126.73, 125.86, 122.90, 122.78, 121.28, 116.92, 116.17, 108.36, 44.95 and 42.37δ.
Embodiment 2
[0118] (5R,6R)-1-benzyl-5-bromo-6-hydroxyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one ( III) 1-benzyl-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one (II, Example 1, 240 g), acetonitrile (1.086 kg), water (227 ml) and Fluoroboric acid (48.5%, 13.4 g) was mixed and cooled to 0 to 5°C. Dibromantin (163.5 g) was homogenized with acetonitrile and added to the reaction mixture. The reaction is carried out at 0 to 5°C for about 3 hours. After the reaction was completed, methyl tert-butyl ether was added in about 45 minutes under the condition that the temperature of the reactor was lower than 10°C. The slurry was cooled to 10-15°C, stirred for 1 hour, then filtered. The product was rinsed with pre-cooled methyl tert-butyl ether, and dried under nitrogen at 40°C to obtain the title compound CMR(CDCl 3 ) 156.0, 137.8, 130.5, 129.6, 129.3, 129.1, 126.6, 123.6, 122.5, 119.6, 110.4, 69.9, 49.6, 47.7, 46.9 and 43.8δ.
Embodiment 3
[0120] (5S, 6S)-1-benzyl-5-bromo-2-oxygen-1,2,5,6-tetrahydro-4H-imidazo[4,5,1-ij]quinolin-6-yl ( 2R)-(6-methoxy-2-naphthyl)propionate (IVA) and (5R,6R)-1-benzyl-5-bromo-2-oxo-1,2,5,6-tetra Hydrogen-4H-imidazo[4,5,1-ij]quinolin-6-yl (2R)-(6-methoxy-2-naphthyl)propionate (IVB) will (5R,6R)- 1-Benzyl-5-bromo-6-hydroxy-5,6 dihydro-imidazo[4,5,1-ij]quinolin-2(1H)-one (III, Example 2, 143g), di Chloromethane (3.136g), N-methylmorpholine (100.2g) and 4-dimethylaminopyridine (497g) were added to the reactor and the mixture was cooled to 0 to 5°C. (R) Naproxen chloride (Formulation 1, 118.5 g) dissolved in dichloromethane (694 ml) was added to the reactor over about 1 hour and the mixture was stirred at 0 to 5°C to complete the reaction. If necessary, additional naproxen chloride was added to complete the reaction. Potassium carbonate diluted with water was added to the mixture. The aqueous phase was extracted with dichloromethane and the combined dichloromethane phases were washed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com