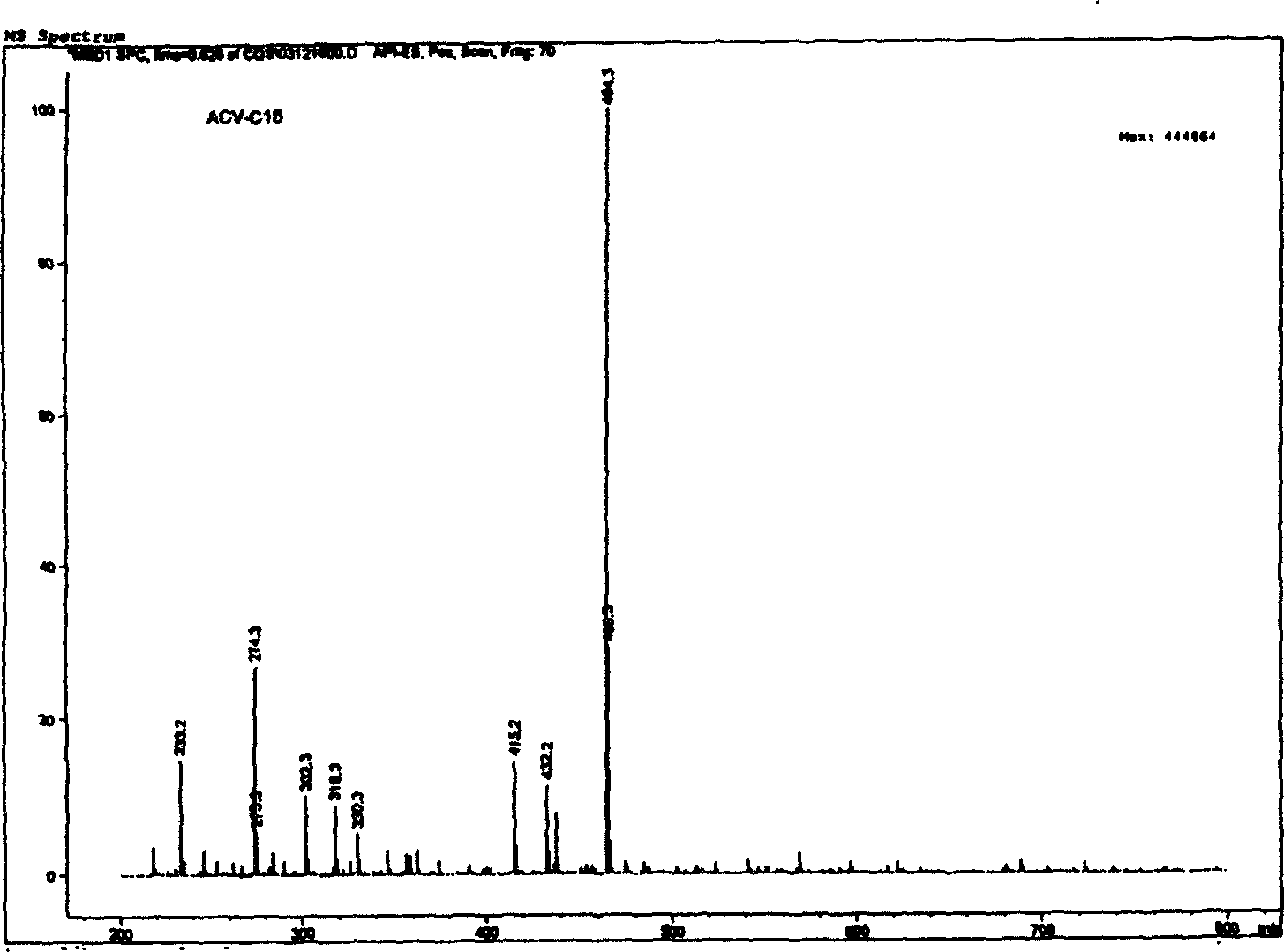
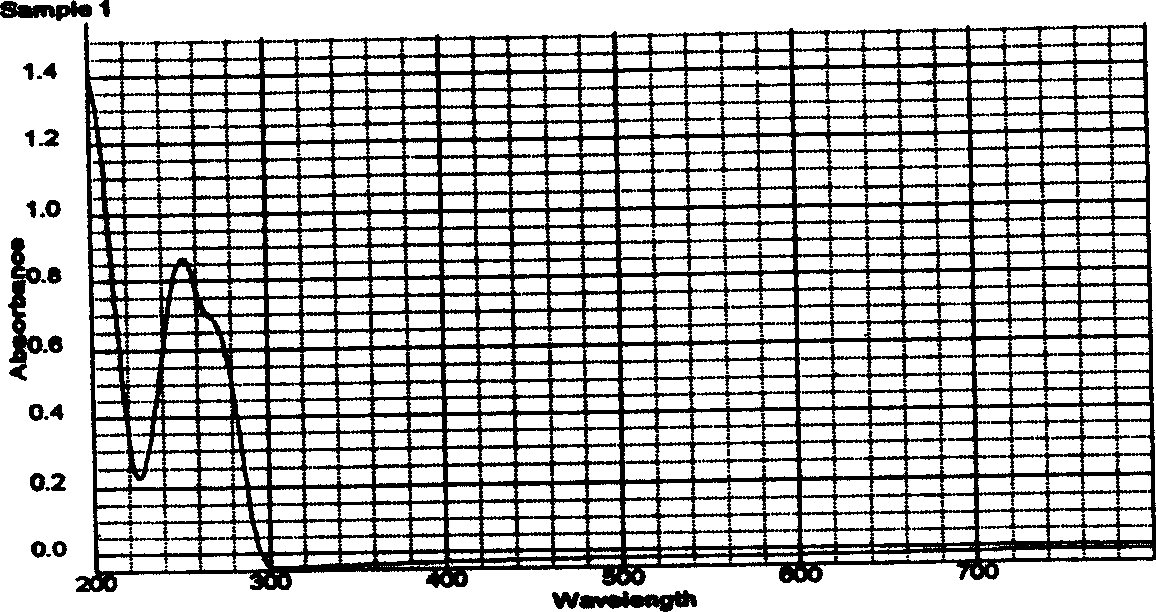
9-(2-fatty ester ethoxy methyl) guanine, liposome, and preparation emthod
A technology of ethoxymethyl and fatty acid ester, which is applied in the field of pharmacy, can solve the problems of limited application and bioavailability of only 15%, and achieve the effect of economical purification method and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 9-(2-palmitate ethoxymethyl)guanine
[0032] (1) Synthesis of 9-(2-palmitate ethoxymethyl)guanine
[0033] Dissolve 2.252 g (10 mmol) of acyclovir in 80 ml of KOH-dried pyridine, stir and add 2.749 g of palmitoyl chloride (10 mmol), seal the above reaction solution in a water bath at 60° C. and stir for continuous reaction for 24 h. After the reaction was completed, 5 times the volume of deionized water was added, and the product was collected by filtration after cooling to room temperature.
[0034] (2) Purification of 9-(2-palmitate ethoxymethyl)guanine
[0035] Suspend the reaction product in 100ml of 0.01M HCl, stir for 30 minutes and then filter, suspend the filtered precipitate in 0.01M NaOH, stir for 10 minutes and then filter, wash the filtrate with 4°C deionized water, dissolve the filtrate with methanol at 60°C to saturation , and then cooled to 4°C to collect; repeat the above operation 5 times and then dry the precipitate to obtain...
Embodiment 2
[0040] Example 2 Preparation of 9-(2-myristate ethoxymethyl)guanine
[0041] (1) Synthesis of 9-(2-myristate ethoxymethyl)guanine
[0042] Dissolve 2.252 grams of acyclovir (10 mmol) in 80 ml of KOH-dried pyridine, add 2.749 grams of myristoyl chloride (10 mmol) to the above solution while stirring, and seal the above reaction solution in a water bath at 60°C and stir continuously. Reaction 24h. After the reaction was completed, 5 times the volume of deionized water was added, and the product was collected by filtration after cooling to room temperature.
[0043] (2) Purification of 9-(2-myristate ethoxymethyl)guanine
[0044] Suspend the reaction product in 100ml of 0.01M HCl, stir for 30 minutes and then filter, suspend the filtered precipitate in 0.01M NaOH, stir for 10 minutes and then filter, wash the filtrate with 4°C deionized water, dissolve the filtrate with methanol at 60°C to saturation , and then cooled to 4°C to collect; after repeating the above operation 5 time...
Embodiment 3
[0045] Example 3 Preparation of 9-(2-laurate ethoxymethyl)guanine
[0046] (1) Synthesis of 9-(2-laurate ethoxymethyl)guanine
[0047] Dissolve 2.252 g of acyclovir (10 mmol) in 80 ml of KOH-dried pyridine, stir and add 2.749 g of lauroyl chloride (10 mmol), seal the above reaction solution in a water bath at 60° C. and stir for continuous reaction for 24 h. After the reaction was completed, 5 times the volume of deionized water was added, and the product was collected by filtration after cooling to room temperature.
[0048] (2) Purification of 9-(2-laurate ethoxymethyl)guanine
[0049] Suspend the reaction product in 100ml of 0.01M HCl, stir for 30 minutes and then filter, suspend the filtered precipitate in 0.01M NaOH, stir for 10 minutes and then filter, wash the filtrate with 4°C deionized water, dissolve the filtrate with methanol at 60°C to saturation , then cooled to 4°C and collected; after repeating the above operation 5 times, the precipitate was dried to obtain 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com