Vitronectin receptor antagonist pharmaceuticals for use in combination therapy
A compound, pharmaceutical technology, applied in the field of new drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
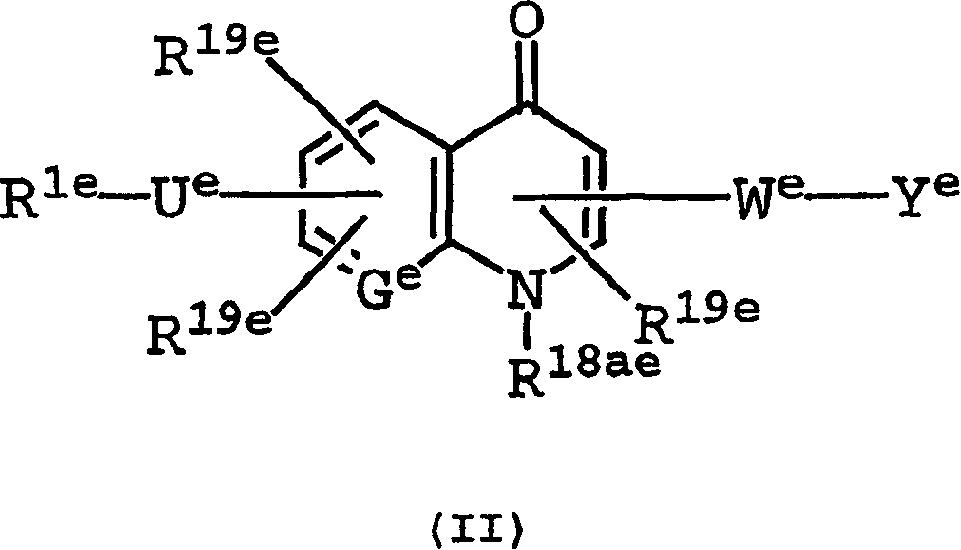
[1568] 2-(((4-(4-(((3-(2-(2-(3-((6-((1-aza-2-(2-sulfophenyl))vinyl) Amino)(3-pyridyl))carbonylamino)propoxy)ethoxy)ethoxy)propyl)amino)sulfonyl)phenyl)phenyl)sulfonyl)amino)-3-((7- ((Imidazol-2-ylamino)methyl)-1-methyl-4-oxo(3-hydroquinolyl)carbonylamino)propionic acid Trifluoroacetate
[1569] Part A - N-(3-(2-(2-(3-aminopropoxy)ethoxy)ethoxy)propyl)(phenylmethoxy)formamide
[1570]4,7,10-trioxa-1,13-tridecanediamine (158ml, 0.72mol), TEA (16.7ml, 0.12mol) and methanol (300ml) were dissolved in peroxide-free THF (1000ml) The solution in was placed in a 3-liter three-necked flask with a nitrogen line equipped with a mechanical stirrer, thermometer, and dropping funnel. The dropping funnel was charged with a solution of benzyl chloroformate (17.1 mL, 0.12 mol) in peroxide-free THF (1000 mL). With rapid stirring, the contents of the dropping funnel were added to the flask over 4 hours while maintaining the temperature below 5°C. The solution was stirred for an additional 30 ...
Embodiment 2
[1613] 3-((7-((imidazol-2-ylamino)methyl)-1-methyl-4-oxo(3-hydroquinolyl))carbonylamino)-2-(((4-(4- (((3-(2-(2-(3-(2-(1,4,7,10-tetraaza-4,7,10-tris(carboxymethyl)cyclododecyl)acetyl Amino)propoxy)ethoxy)ethoxy)propyl)amino)sulfonyl)phenyl)phenyl)sulfonyl)amino)propionic acid Bis(trifluoroacetate)
[1614] Part A - Benzyl 2-(1,4,7,10-tetraaza-4,7,10-tris(((tert-butyl)oxycarbonyl)methyl)cyclododecyl)acetate
[1615] Stirring (1,4,7,10-tetraaza-4,7-bis(((tert-butyl)oxycarbonyl)methyl)cyclododecyl)acetic acid at ambient temperature under nitrogen atmosphere A solution of tert-butyl ester (0.9222g, 1.79mmol), TEA (1.8ml) and benzyl bromoacetate (0.86ml, 5.37mmol) in anhydrous DMF (24ml) for 24 hours. DMF was removed in vacuo, and the resulting oil was dissolved in EtOAc (300ml). The solution was washed sequentially with water (2 x 50ml) and saturated sodium chloride (50ml), dried (magnesium sulfate) and concentrated to give the title compound (1.26g) as an amorphous solid. MS: ...
Embodiment 3
[1632] 2-(((4-(3-(N-(3-(2-(2-(3-((6-((1-aza-2-(2-sulfophenyl)vinyl)amino) (3-pyridyl))carbonylamino)propoxy)ethoxy)ethoxy)propyl)carbamoyl)propoxy)-2,6-dimethylphenyl)sulfonyl)amino)- 3-((7-((imidazol-2-ylamino)methyl-1-methyl-4-oxo(3-hydroquinolyl))carbonylamino)propanoic acid Trifluoroacetate
[1633] Part A - Ethyl 4-(3,5-dimethylphenoxy)butanoate
[1634]Sodium metal (17.12 g, 0.744 mmol) was added to absolute ethanol (350 ml), and stirred until dissolved. 3,5-Dimethylphenol was added, and the solution was stirred at ambient temperature for 15 minutes. Ethyl 4-bromoacetate (58.7ml, 0.41mol) was added and the solution was stirred at ambient temperature under nitrogen for 28 hours. Ethanol was removed in vacuo, and the oily solid was partitioned between water (1 L) and EtOAc (500 ml). The aqueous layer was extracted with additional EtOAc (500ml). The combined EtOAc extracts were washed sequentially with saturated sodium bicarbonate (300ml) and saturated sodium chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com