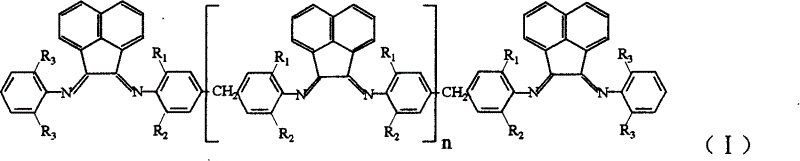
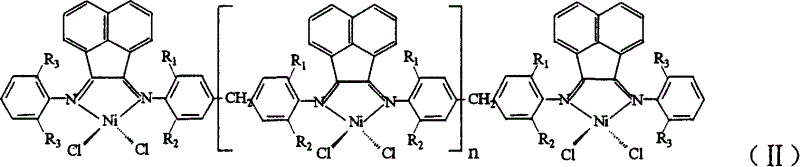
Multinuclear acenaphthene diimine nickle catalyst for synthesis of branched polyethylene
A technology of nuclear acenaphthylene diimide nickel chloride and branched polyethylene is applied in the field of polynuclear acenaphthylene diimide nickel chloride complex catalyst and its preparation field, and can solve the problems of irregular product shape, difficulty in promotion and use, and high cost , to achieve significant advantages and the effect of promoting the use of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Nickel complex (Ni) n+2 L 1 Cl 2(n+2) Preparation of polynuclear acenaphthylenediimide nickel complex can be obtained by the following method: under the protection of nitrogen, dissolve 0.5354g (4.14mmol) anhydrous nickel chloride in 20ml absolute ethanol for 2 to 3 hours, and after the dissolution , was added to dissolve 2.005g (4.14mmol) ligand L 1 30 g CH 2 Cl 2 solution, reflux reaction for 12 to 16 hours, drained to remove the solvent, washed three times with 30ml of anhydrous ether, and dried in vacuum to obtain the desired nickel complex (Ni) n+2 L 1 Cl 2(n+2) .
[0027] 2. Catalyst preparation:
[0028] 2-1. Mix 5 g of microspherical SiO 2 Put it in a tube furnace, heat it under nitrogen, raise the temperature to 600°C, calcine and dehydrate at a constant temperature, cool after 6 hours, and discharge under nitrogen protection to obtain 4 grams of SiO 2 , placed in a reaction flask, add 40ml of heptane, under nitrogen protection and constant stirrin...
Embodiment 2
[0037] 1. In Step 1 of Example 1, 4.14 mmol of ligand L 1 Change to 4.14mmol ligand L 2 , and the rest of the reaction conditions are the same, the complex (Ni) n+2 L 2 Cl 2(n+2) .
[0038] 2. In embodiment one step 2-4, will add 1.624g nickel complex (Ni) n+2 L 1 Cl 2(n+2) CH 2 Cl 2 solution, add 1.486g (Ni) instead n+2 L 2 Cl 2(n+2) CH 2 Cl 2 solution, all the other conditions and operations are the same as in Example One, and the prepared catalyst is polymerized under the same conditions as in Example One.
[0039] The density of the obtained branched polyethylene under the above polymerization conditions is 0.894g / cm 3 , The degree of branching is 68.0 elastomer. The catalytic efficiency of the catalyst is 65kgLLDPE / molNi.
Embodiment 3
[0041] 1. In Step 1 of Example 1, 2.005g (4.14mmol) of ligand L 1 Change to 1.788 (4.14mmol) Ligand L 3 , and the rest of the reaction conditions are the same, the complex (Ni) n+2 L 3 Cl 2(n+2) .
[0042] 2. In embodiment one step 2-4, 1.624g nickel complex (Ni) n+2 L 1 Cl 2(n+2) Change to 1.577g(Ni) n+2 L 3 Cl 2(n+2) , all the other conditions and operations are the same as in Example 1, and the prepared catalyst is polymerized under the same conditions as in Example 1.
[0043] The density of the branched polyethylene prepared under the above polymerization conditions is 0.887g / cm 3 , The degree of branching is 50.2 elastomers. The catalytic efficiency of the catalyst is 65kgLLDPE / molNi.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com