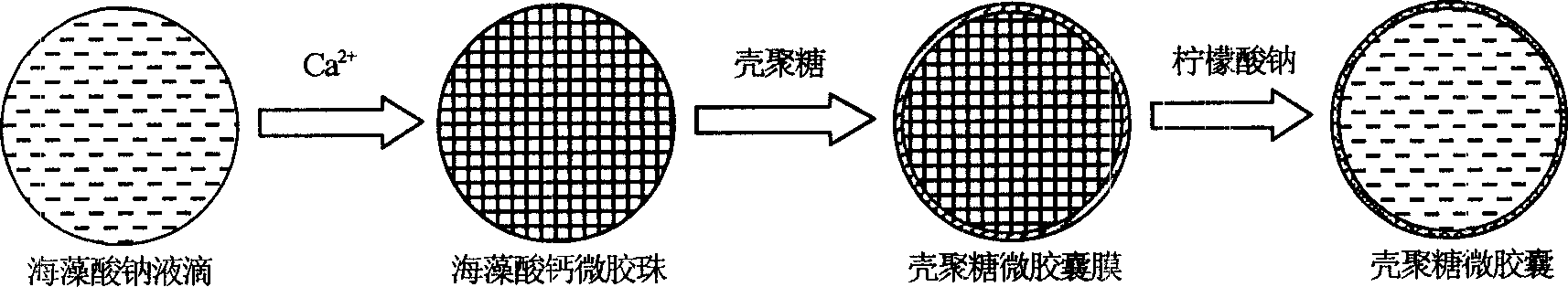
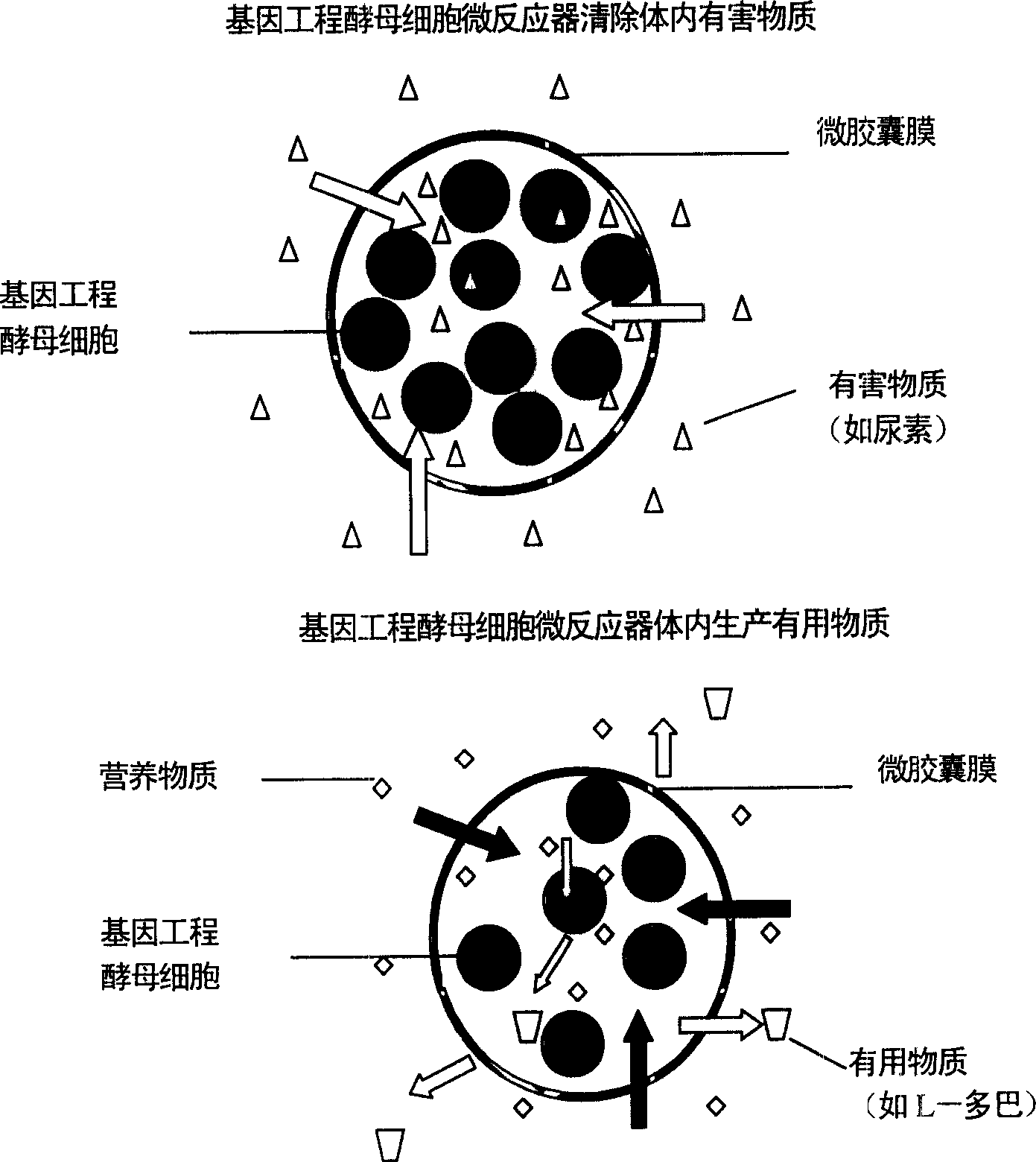
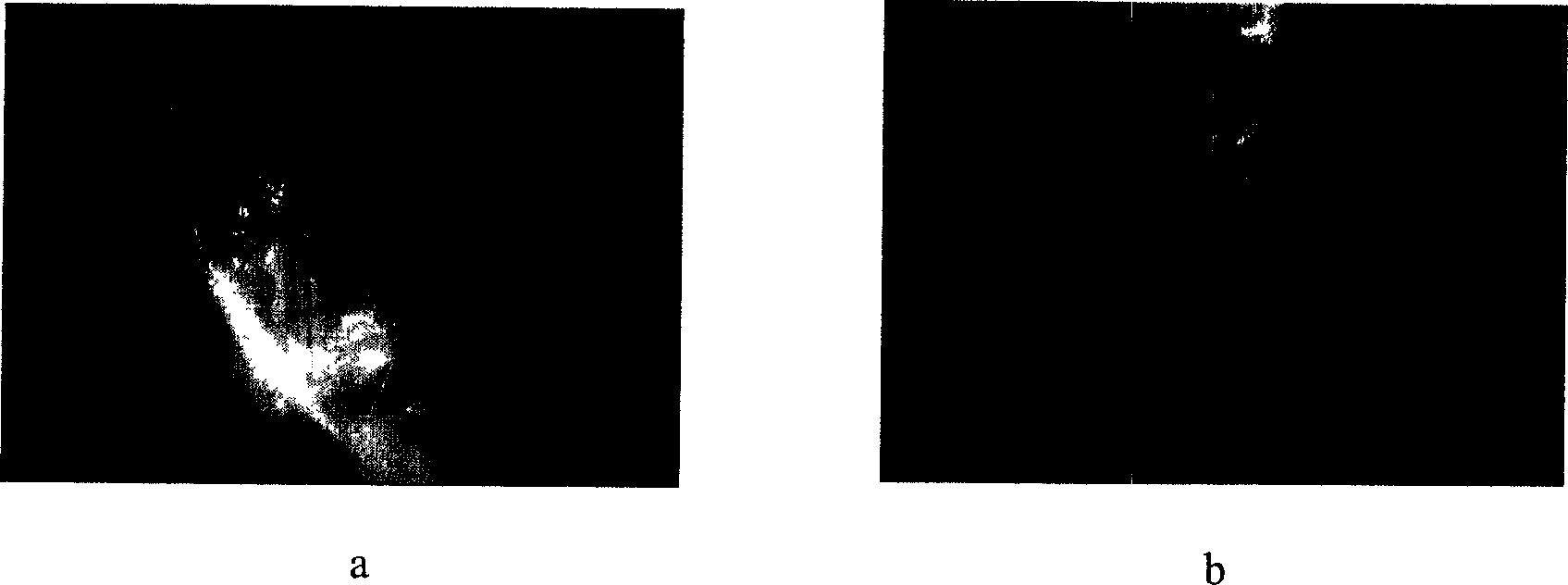Microencapsulated saccharomyces and its application
A yeast and microencapsulation technology, applied in microcapsules, fungi, digestive system, etc., can solve the problems of poor stability, high cost, short half-life in vivo, etc., and achieve good biocompatibility and good intestinal adhesion performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Example 1: ACA microcapsules containing yeast were prepared under physiological conditions. During the preparation process, the survival rate of yeast was 100%, and the yeast content was 10%. 8 One / ml microcapsules, the particle size of the microcapsules is precisely controllable in the range of 100-1000μm.
example 2
[0018] Example 2: After oral perfusion of ACA microcapsules containing indicator dyes in mice, the mice were killed 2 hours later and the stomach wall was dissected, and the complete microcapsules were seen. (See image 3 ).
example 3
[0019] Example 3: ACA microcapsules containing bovine hemoglobin showed zero-order release in simulated gastric fluid hemoglobin, and first-order release curve in simulated intestinal fluid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com