Preparation of gentian effective part and its application
A technology of effective parts and gentian, applied in the field of preparation of effective parts of traditional Chinese medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0067] Example 1, Preparation Example 1 of the effective fraction of Gentiana composed of loganic acid and gentiopicroside
[0068] Take 100g of gentian crude powder, add 400mL of 30% ethanol solution, reflux for 1 hour, repeat the extraction twice, filter out the extract, combine, recover to 100mL under reduced pressure, refrigerate for 1 day, centrifuge, and take the supernatant , added to a chromatographic column equipped with 300mL HP20 filler, rinsed with 500mL deionized water, then rinsed with 60% ethanol solution, collected 600mL of ethanol eluate, evaporated to dryness under reduced pressure, and vacuum dried to obtain 7.6g of the effective fraction of Gentiana.
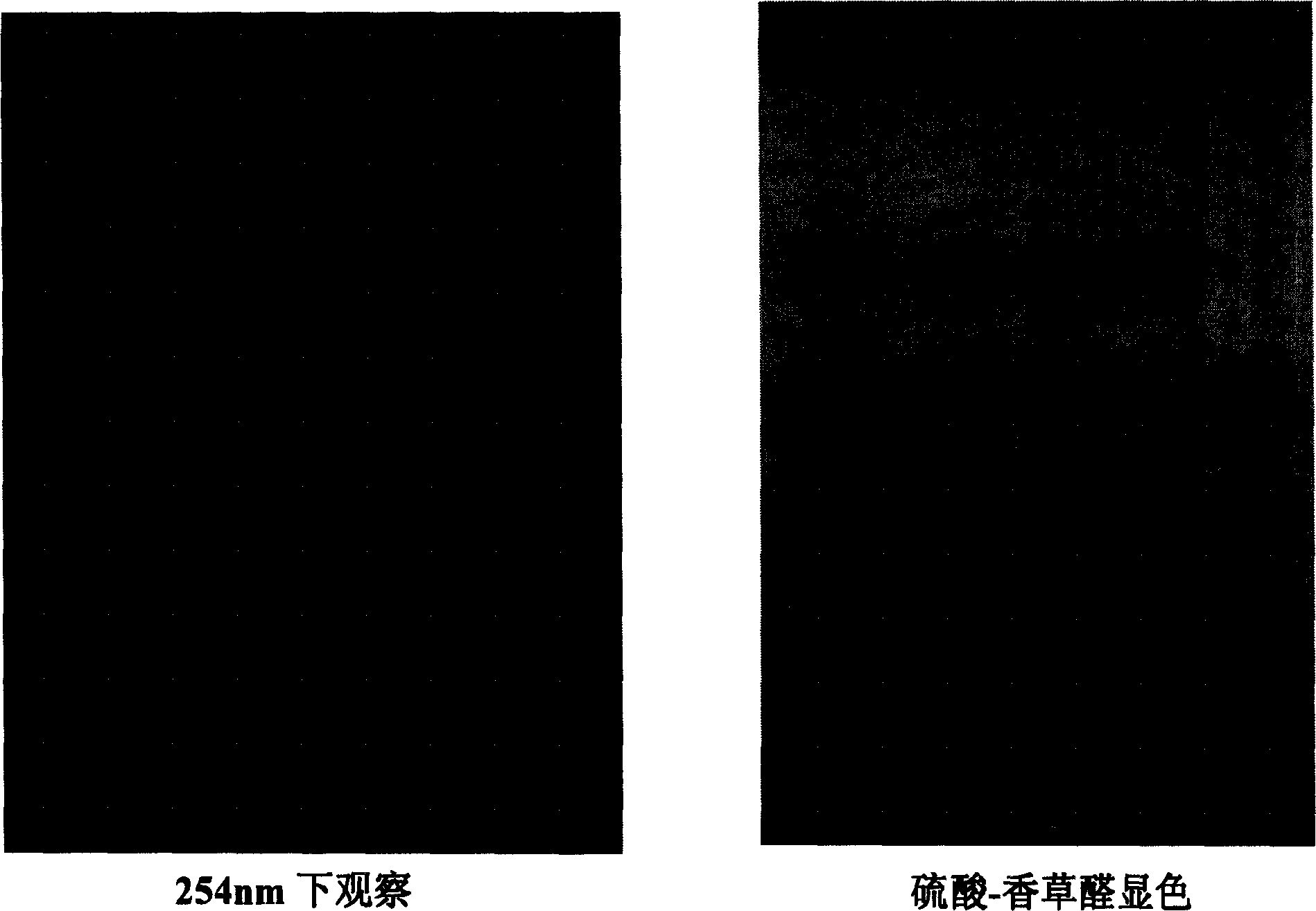
[0069] The active part of gentian obtained by TLC can be seen to have clear spots at the same Rf value as the standard products loganic acid and gentiopicroside;
[0070] The content of loganic acid and gentiopicroside was determined by HPLC, and the content of the two main compounds accounted for 66.54% of t...
example 2
[0071] Example 2, take 100g of gentian crude powder, add 400mL of 40% acetone solution, reflux for 1 hour, repeat the extraction 3 times, filter the extract, combine, recover to 100mL under reduced pressure, refrigerate for 2 days, centrifuge, take Add the supernatant to a chromatographic column equipped with 300mL HP20 filler, rinse with 500mL deionized water, then rinse with 60% ethanol solution, collect 600mL of ethanol eluate, evaporate to dryness under reduced pressure, and dry in vacuum to obtain the effective fraction of Gentiana 6.2 g.
[0072] The active part of gentian obtained by TLC can be seen to have clear spots at the same Rf value as the standard products loganic acid and gentiopicroside;
[0073] The content of loganic acid and gentiopicroside was determined by HPLC, and the content of the two main compounds accounted for 90.54% of the solid content of the effective part.
example 3
[0074] Example 3, the preparation example of the powder injection of the active part of Gentiana composed of loganic acid and gentiopicroside
[0075] Powder Injection Prescription:
[0076] Active parts of gentian 300g
[0077] Mannitol 300g
[0078] Add water for injection to 4000mL
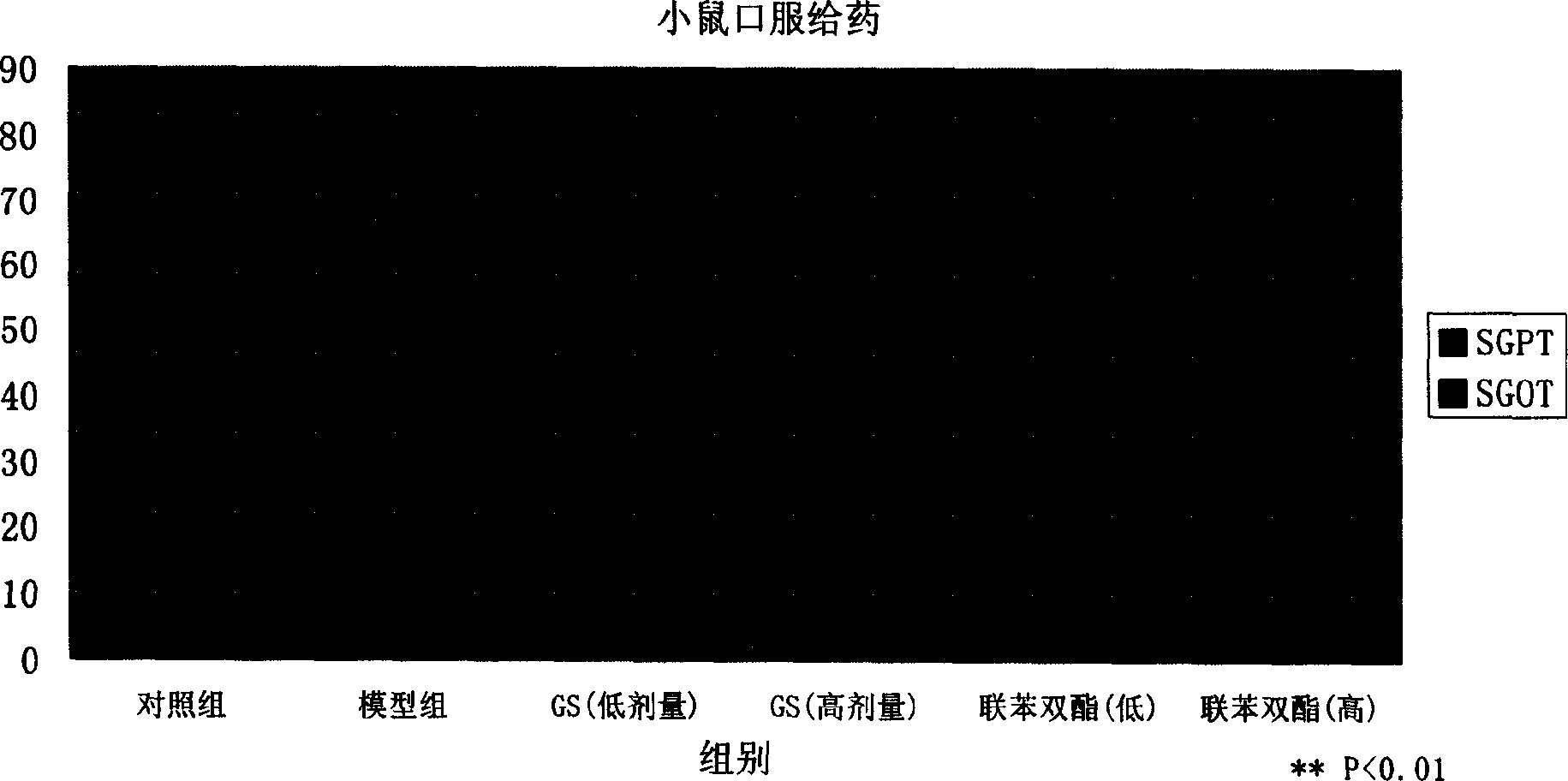
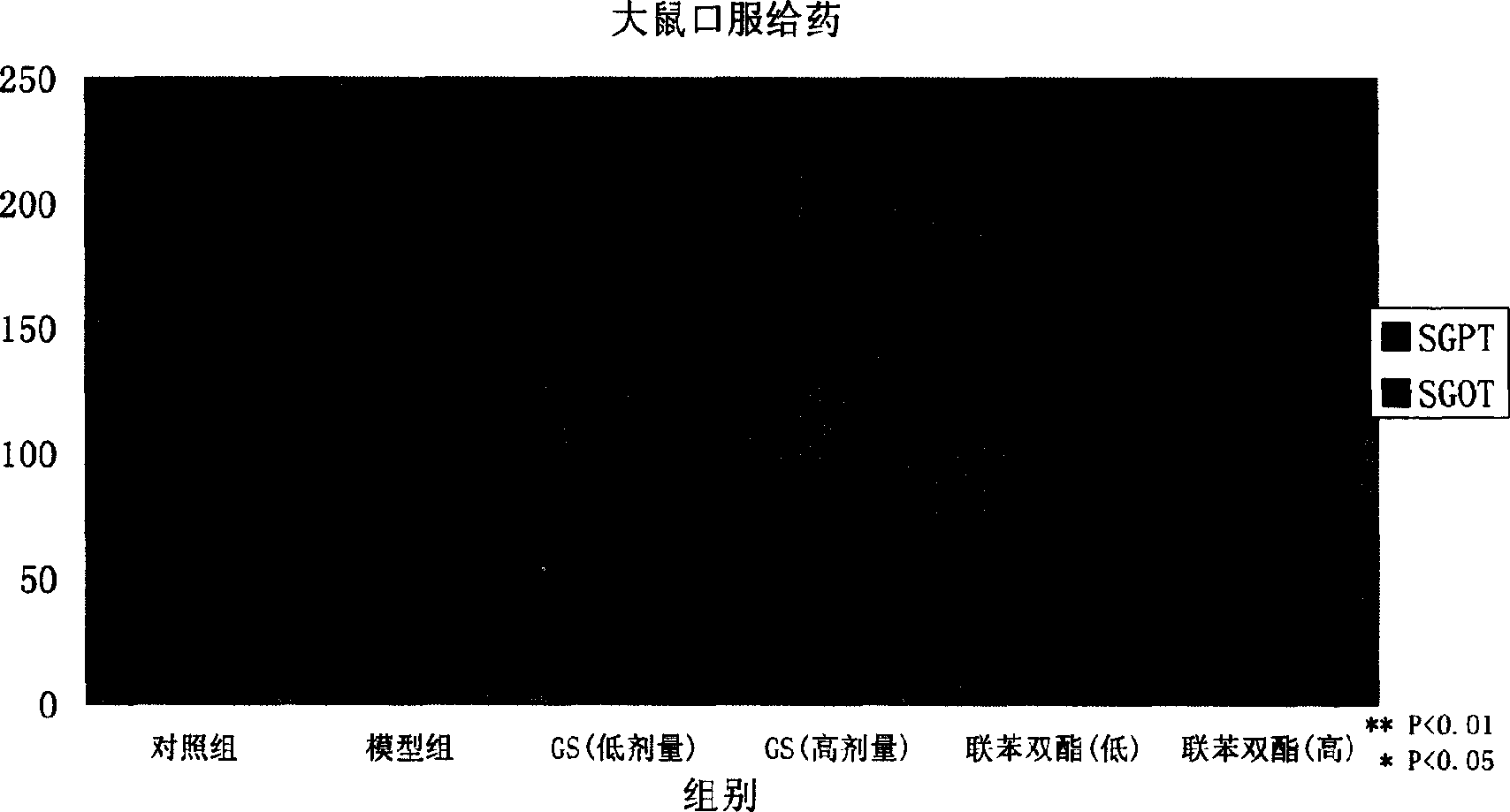
[0079] Preparation method: According to the above prescription, dissolve the active part of gentiana and mannitol in water for injection, add 4g of activated carbon, boil at 100°C for 15 minutes, and let it stand for 24 hours; filter, add dilute hydrochloric acid to adjust the pH to 7.0; dispense in 10ml of penicillin In a vial, 4 mL each; freeze-dry in a freeze dryer at -40°C for 5 hours; sublimate at -15°C-20°C for a total of 24 hours; dry at 40°C for 2 hours; press cap and seal.
[0080] Properties: This product is a light yellow lump
[0081] Quality index: each preparation contains 300 mg of active part of gentian, and each contains no less than 150 mg of loganic acid and gentiopicrosi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com