Stable stored composite emulsion carrier in even dimension for hydrophilicity medication and preparation method
A hydrophilic drug, homogeneous technology for emulsion delivery, oil/fat/wax non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 (add the mixture of lecithin and cholesterol in the oil phase)
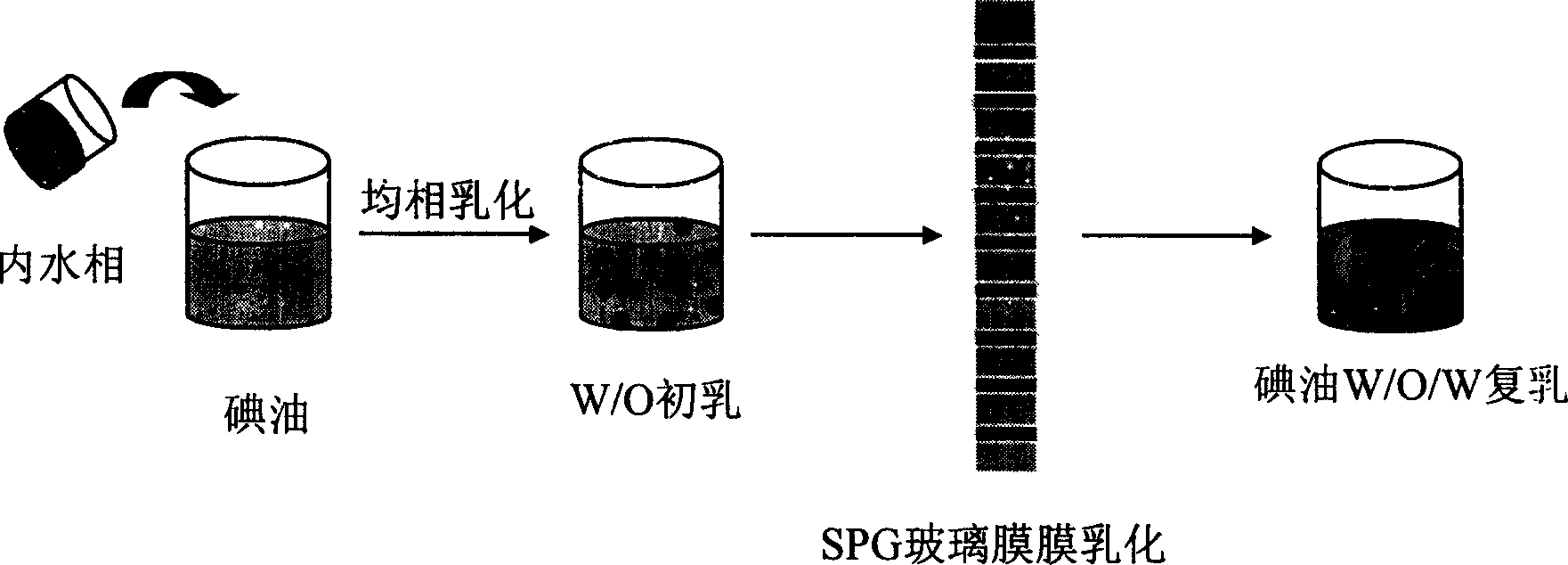
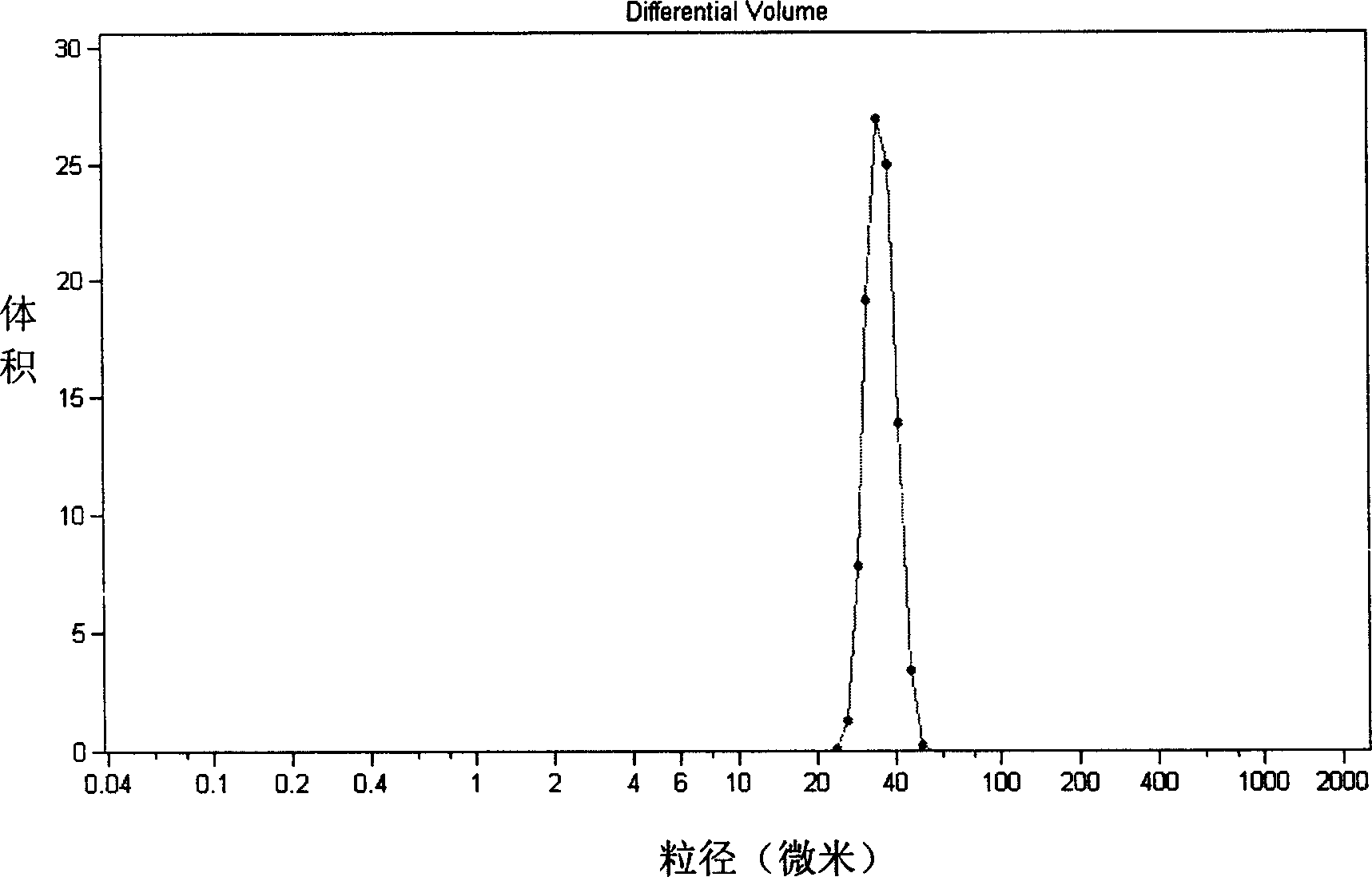
[0034] The microporous membrane with a pore size of 7 μm is soaked in a hydrophilic substance to fully wet the porous membrane. 2.5 ml of glucose aqueous solution of mitomycin C was prepared, the glucose concentration was 5.8 wt %, and the amount of mitomycin C added was 2.67 mg / ml water. The aqueous solution is mixed with 5.0 ml of lipiodol (cholesterol content: 0.1-2.0 wt %) dissolved with lecithin and cholesterol at a molar ratio of 1:1, and the volume ratio of the oil-water phase is 2:1. Emulsify with a homogeneous emulsifier for 3 minutes to obtain W / O colostrum. Add the water-soluble suspension stabilizer and emulsifier into 100ml of water, stir until completely dissolved as the external water phase. Use 0.085Kgf / cm of 8.0g of W / O type colostrum 2 The constant pressure is pressed into the outer water phase through the hydrophilic microporous membrane with uniform pore size to obtain t...
Embodiment 2
[0039] Embodiment 2 (add the mixture of lecithin and cholesterol in the oil phase)
[0040] The microporous membrane with a pore size of 7 μm is soaked in a hydrophilic substance to fully wet the porous membrane. An aqueous glucose solution of mitomycin C was prepared, the glucose concentration was 5.8 wt%, and the amount of mitomycin C added was 2.4 mg / ml water. This aqueous solution is mixed with 5 ml of super liquefied iodized oil (dissolved with oil-soluble emulsifier) that is dissolved in lecithin and cholesterol (cholesterol content 0.4%) with a molar ratio of 2:1, and the volume ratio of the oil-water phase is 3:1. Emulsify with a homogeneous emulsifier for 3 minutes to obtain W / O colostrum. Add the water-soluble suspension stabilizer and emulsifier into 100ml of water, stir until completely dissolved as the external water phase. Use 0.085Kgf / cm of 8.0g of W / O type colostrum 2 The constant pressure is pressed into the outer water phase through the hydrophilic micro...
Embodiment 3
[0042] Embodiment 3 (in the internal aqueous phase, add internal albumin)
[0043] The microporous membrane with a pore size of 7 μm is soaked in a hydrophilic substance to fully wet the porous membrane. The glucose aqueous solution of doxorubicin was prepared, the glucose concentration was 5.8wt%, the added concentration of bovine serum albumin was 0.5wt%, and the added amount of doxorubicin was 12.0mg / ml water. The aqueous solution was mixed with 5 ml of lipiodol (dissolved with an oil-soluble emulsifier), and the volume ratio of the oil-water phase was 3:1. Emulsify with a homogeneous emulsifier for 3 minutes to obtain W / O colostrum. Add 1.0 g of water-soluble suspension stabilizer and emulsifier into 100 ml of water, stir until completely dissolved as the external water phase. Use 0.085Kgf / cm of 8.0g of W / O type colostrum 2 The constant pressure is pressed into the outer water phase through the hydrophilic microporous membrane with uniform pore size to obtain a W / O / W ty...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com