Preparation method of silver chloride
A technology of silver chloride and silver solution, applied in the field of material chemistry, can solve the problems of high cost, inability to regulate and control the catalytic activity of silver chloride, and high production cost of silver chloride, so as to prevent particle agglomeration, realize catalytic activity, and reduce the consumption of raw materials. effect used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of silver chloride of the present invention, comprises the following steps:
[0032] (1) Prepare 20 mL of silver nitrate solution with a concentration of 0.03 mol / L, add concentrated ammonia water with a mass percentage concentration of 25% to 28% to prepare silver ammonia solution, and adjust its pH value to 11.0 for subsequent use;
[0033] (2) the silver ammonia solution prepared by step (1) and cetyltrimethylammonium chloride solution (cetyltrimethylammonium chloride is 2 times of the amount of substance of silver nitrate) are mixed;
[0034] (3) Transfer the mixed solution after step (2) to a 50mL autoclave with a polytetrafluoroethylene liner, and react at 120°C for 8 hours;
[0035] (4) After the reaction product after step (3) was naturally cooled to room temperature, the sample was centrifuged, washed with deionized water and ethanol for 3 times, and finally vacuum-dried for 8 hours to obtain the silver chloride product.
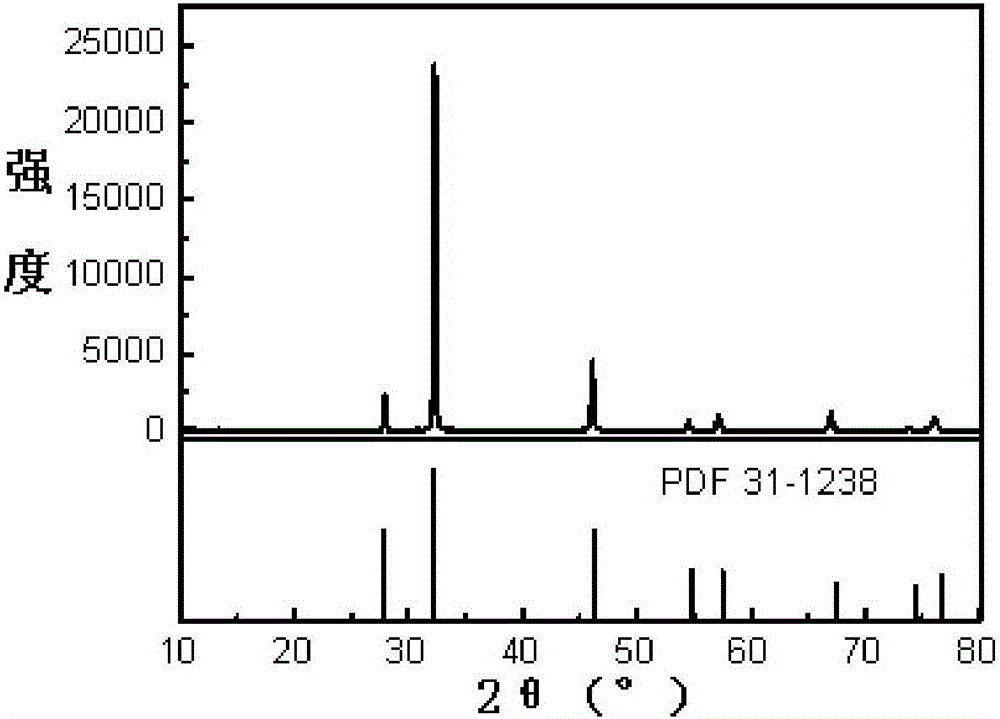
[0036] Test the ...
Embodiment 2
[0038] A preparation method of silver chloride of the present invention, comprises the following steps:
[0039] (1) Prepare 20 mL of silver nitrate solution with a concentration of 0.03 mol / L, add concentrated ammonia water with a mass percentage concentration of 25% to 28% to prepare silver ammonia solution, and adjust its pH value to 11.0 for subsequent use;
[0040] (2) the silver ammonia solution prepared by step (1) and cetyltrimethylammonium chloride solution (cetyltrimethylammonium chloride is 2 times of the amount of substance of silver nitrate) are mixed;
[0041] (3) Transfer the mixed solution after step (2) to a 50mL autoclave with a polytetrafluoroethylene liner, and react at 120°C for 15min;
[0042] (4) After the reaction product after step (3) was naturally cooled to room temperature, the sample was centrifuged, washed with deionized water and ethanol for 3 times, and finally vacuum-dried for 8 hours to obtain the silver chloride product.
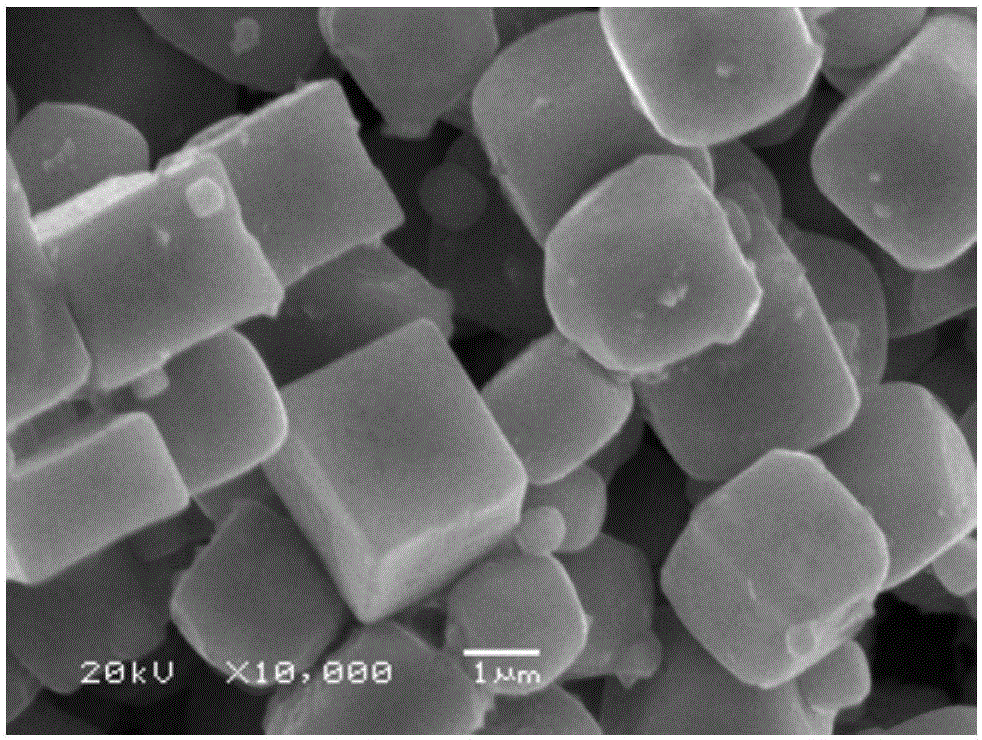
[0043] The SEM figur...
Embodiment 3
[0045] A preparation method of silver chloride of the present invention, comprises the following steps:
[0046] (1) Prepare 10 mL of silver acetate solution with a concentration of 0.01 mol / L, adjust its pH value to 4.0 with dilute nitric acid, and set aside;
[0047] (2) the silver acetate solution prepared by step (1) and 25mL p-chlorophenol solution (p-chlorophenol is 20 times of the amount of silver acetate substance) are mixed uniformly;
[0048] (3) Transfer the mixed solution after step (2) to a 50mL autoclave with a polytetrafluoroethylene liner, and react at 140°C for 16 hours;
[0049] (4) After the reaction product after step (3) was naturally cooled to room temperature, the sample was centrifuged, washed with deionized water and ethanol for 3 times, and finally vacuum-dried for 8 hours to obtain the silver chloride product.
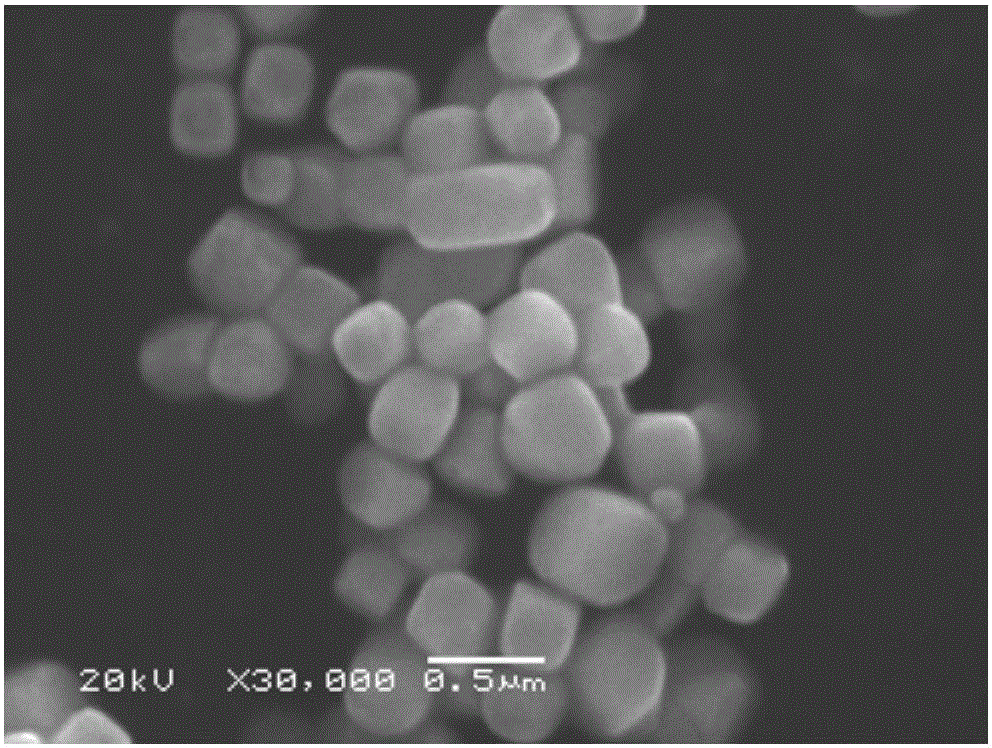
[0050] The SEM figure of present embodiment gained silver chloride product is as Figure 4 shown by Figure 4 It can be seen that the obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com