Method for producing large dicalcium phosphate crystals by utilizing seed crystals and returned material
A calcium hydrogen phosphate, large crystal technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry and other directions, can solve the problems of increased labor intensity of packaging, difficult control of unit weight, difficult solid-liquid separation, etc., to improve the packaging environment, The effect of reducing the manpower of packaging and improving the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
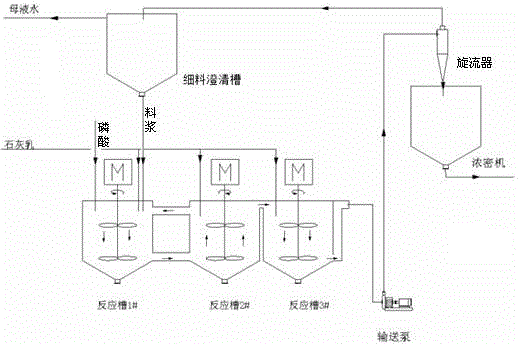
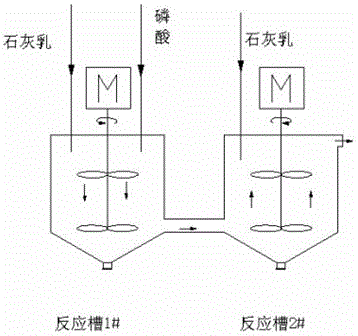
[0017] (1) In the conventional production process of calcium hydrogen phosphate, the neutralization reaction first tank and the second neutralization reaction tank are connected by adding lime milk to adjust the pH, and then the neutralization reaction is carried out according to the return ratio L / L of 1:12. Part of the slurry in the second tank is returned to the first tank of neutralization reaction by stirring, thereby reducing the supersaturation of the first tank of neutralization reaction and increasing the crystal growth time; controlling the pH of the first tank of neutralization reaction to 4.0, and the second tank of neutralization reaction The pH is 4.8, which creates a good environment for the crystallization process, and the growth rate is accelerated at this time;
[0018] (2) Put the slurry reacted in the second tank of neutralization reaction in step (1) into the third tank of neutralization reaction, and add milk of lime to adjust the pH to 6.3. At this time, ...
Embodiment 2
[0022] (1) In the conventional production process of calcium hydrogen phosphate, the first neutralization reaction tank and the second neutralization reaction tank are connected by adding lime milk to adjust the pH, and then the neutralization reaction is carried out according to the return ratio L / L of 1:15. Part of the slurry in the second tank is returned to the first tank of neutralization reaction by stirring, thereby reducing the supersaturation of the first tank of neutralization reaction and increasing the crystal growth time; controlling the pH of the first tank of neutralization reaction to 3.5, and the second tank of neutralization reaction The pH is 4.5, which creates a good environment for the crystallization process, and the growth rate is accelerated at this time;
[0023] (2) Put the slurry reacted in the second tank of the neutralization reaction in step (1) into the third tank of the neutralization reaction, and add milk of lime to adjust the pH to 6.0. At thi...
Embodiment 3
[0027] (1) In the conventional production process of calcium hydrogen phosphate, the neutralization reaction first tank and the second neutralization reaction tank are connected by adding lime milk to adjust the pH, and then the neutralization reaction is carried out according to the return ratio L / L of 1:20. Part of the slurry in the second tank is returned to the first tank of neutralization reaction by stirring, thereby reducing the supersaturation of the first tank of neutralization reaction and increasing the crystal growth time; controlling the pH of the first tank of neutralization reaction to 3.6, and the second tank of neutralization reaction The pH is 4.6, which creates a good environment for the crystallization process, and the growth rate is accelerated at this time;
[0028] (2) Put the slurry reacted in the second tank of neutralization reaction in step (1) into the third tank of neutralization reaction, and add milk of lime to adjust the pH to 6.2. At this time, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com