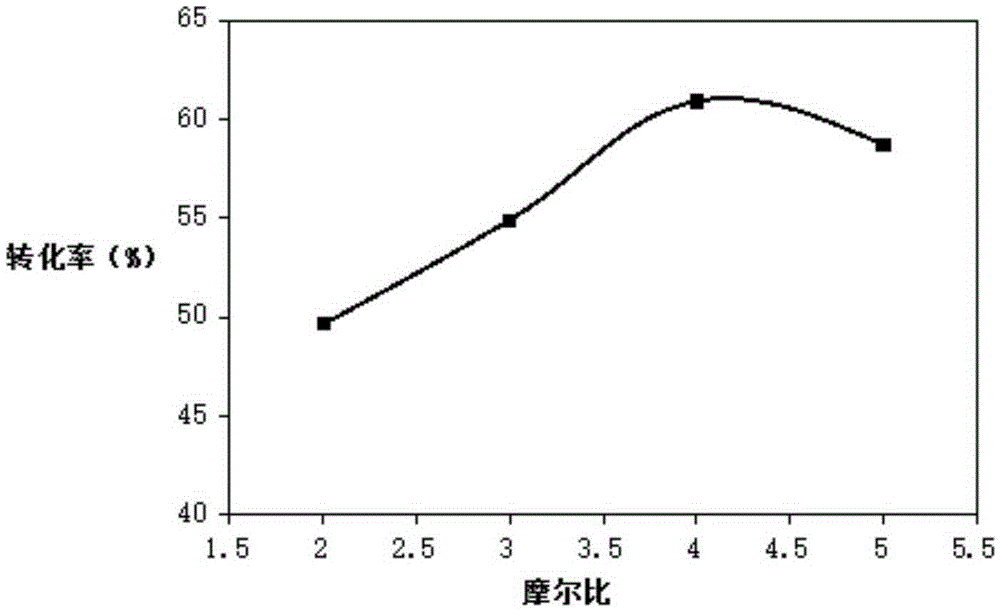
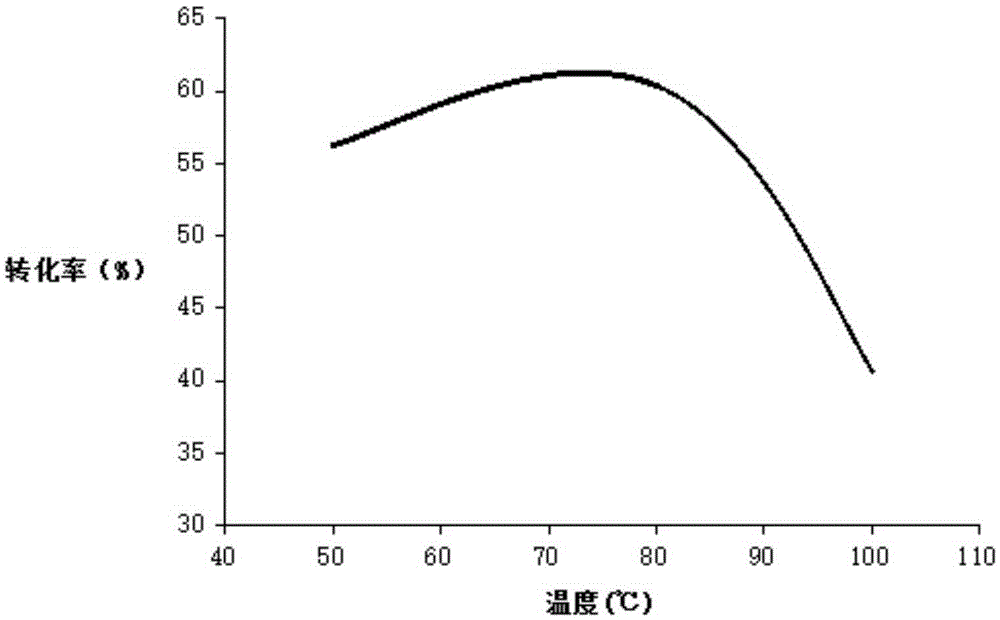
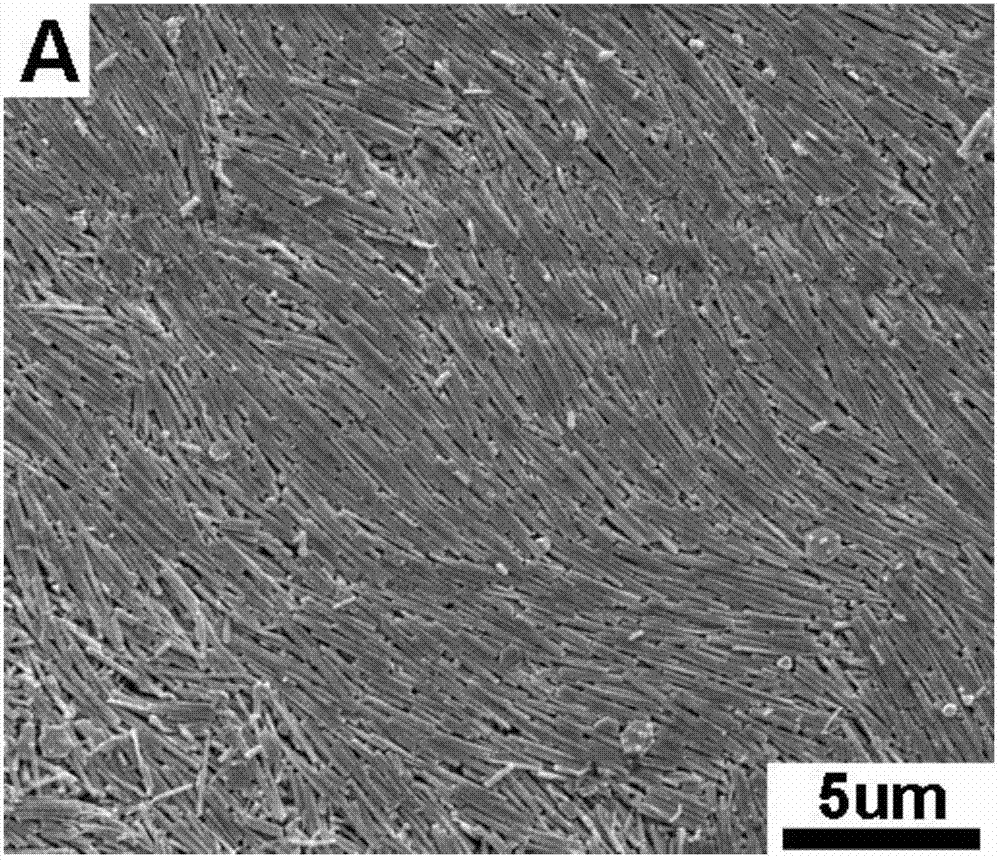
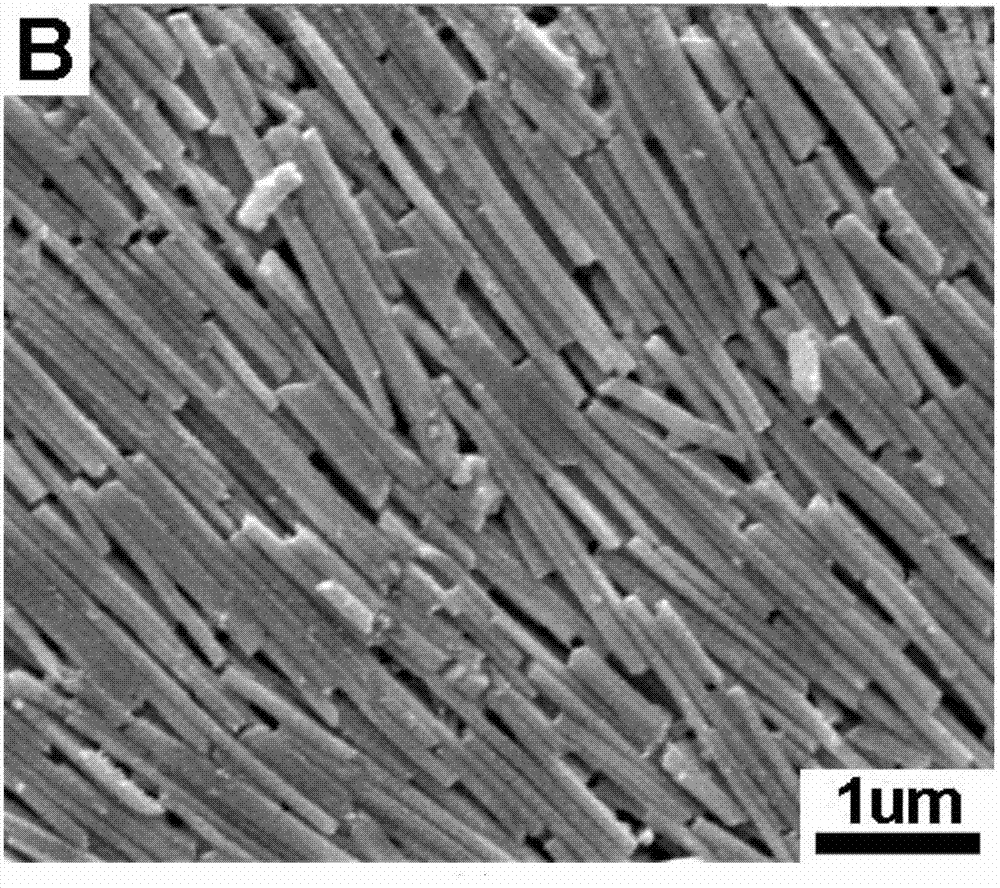
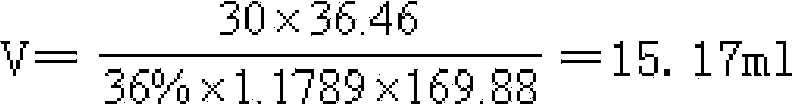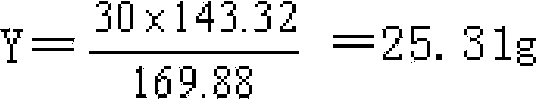Patents
Literature
106results about "Silver halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
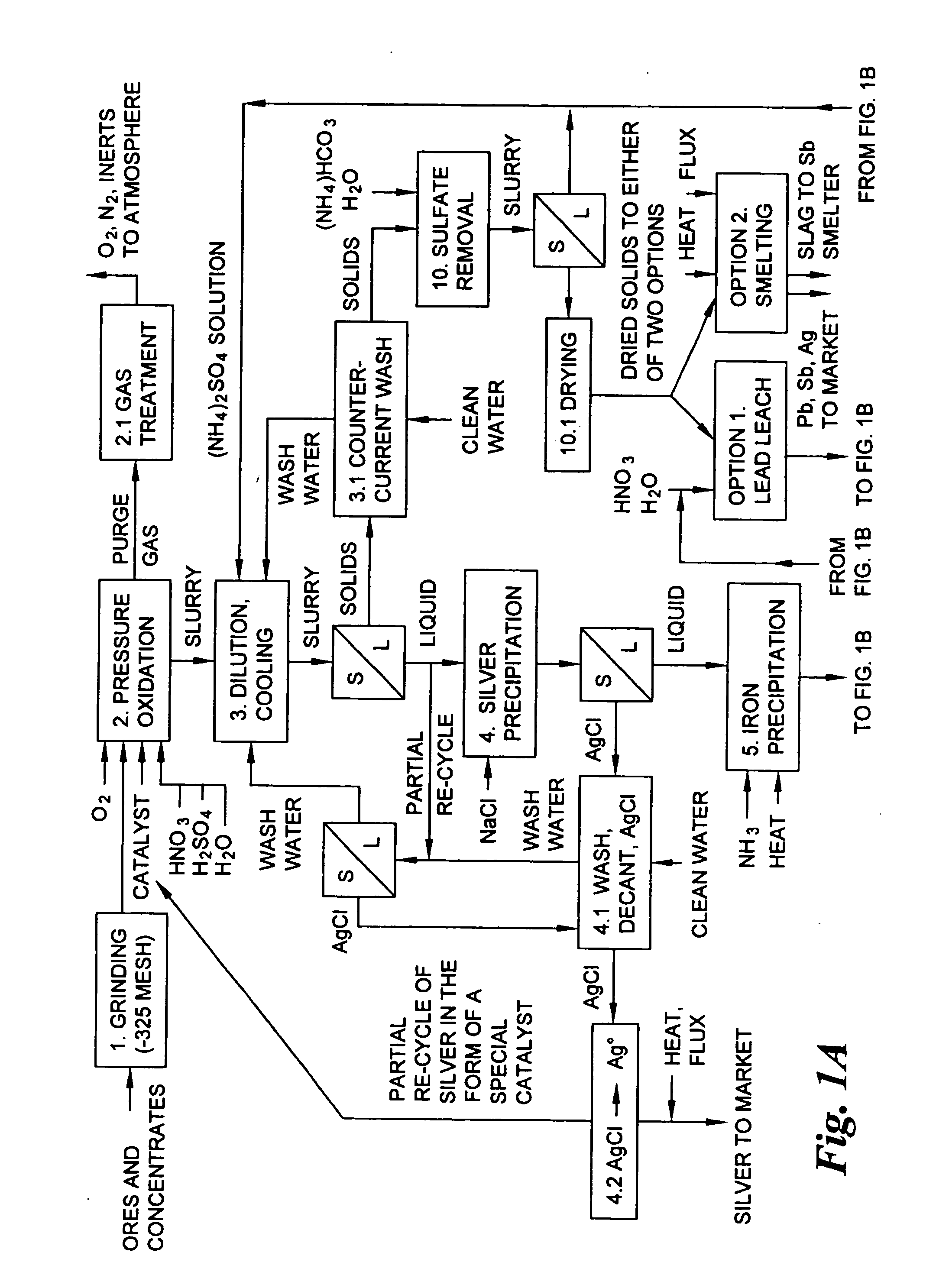
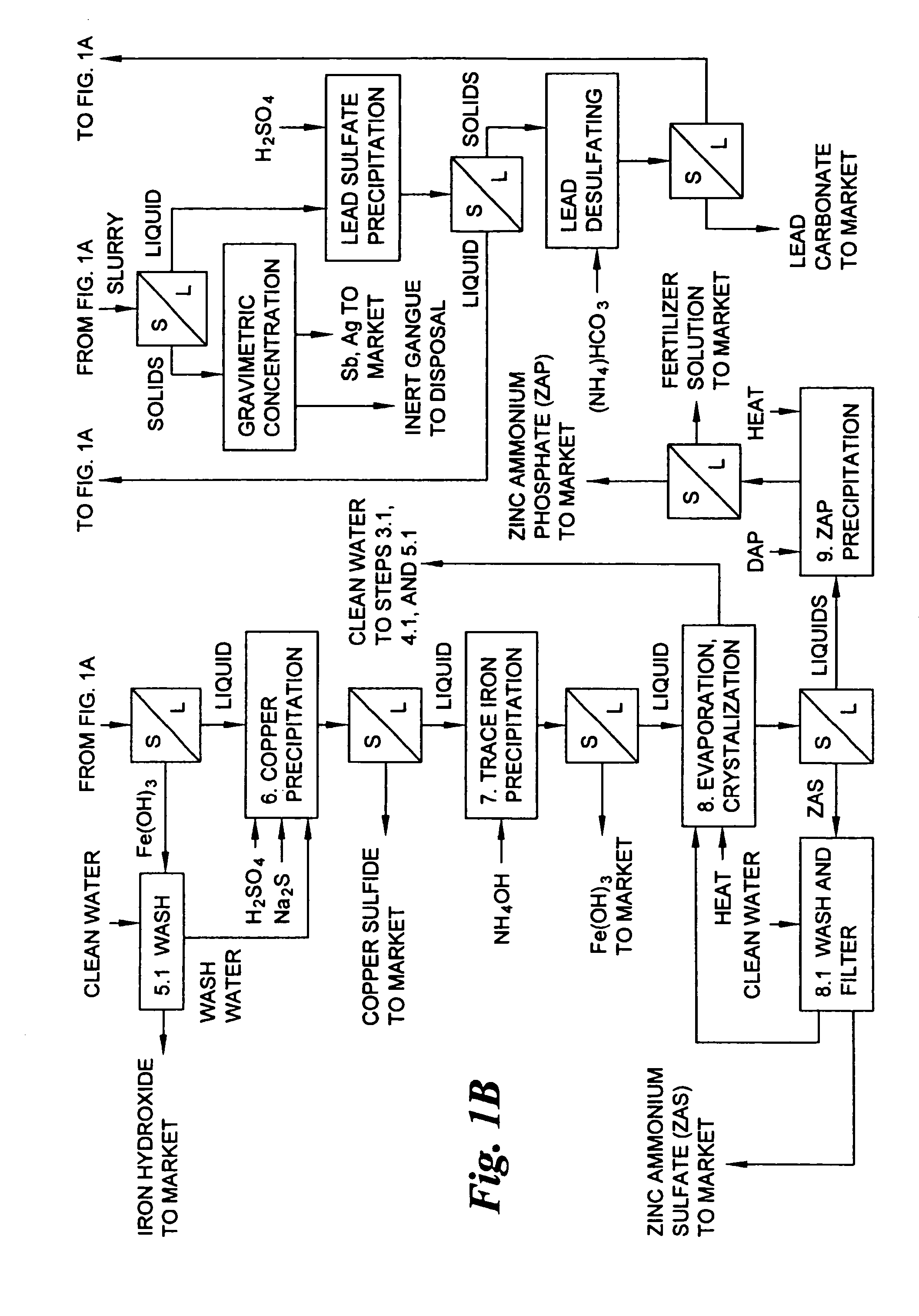
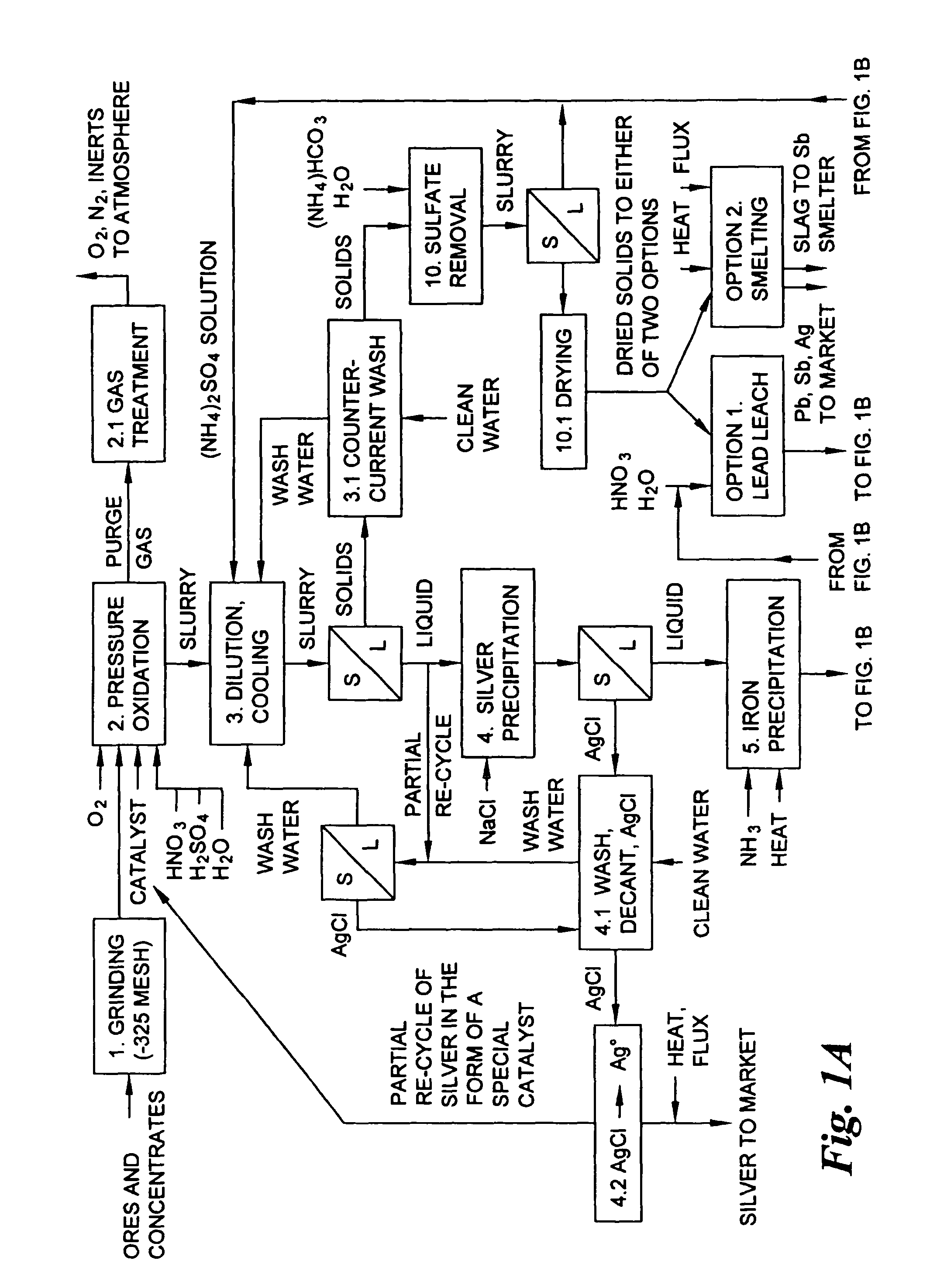
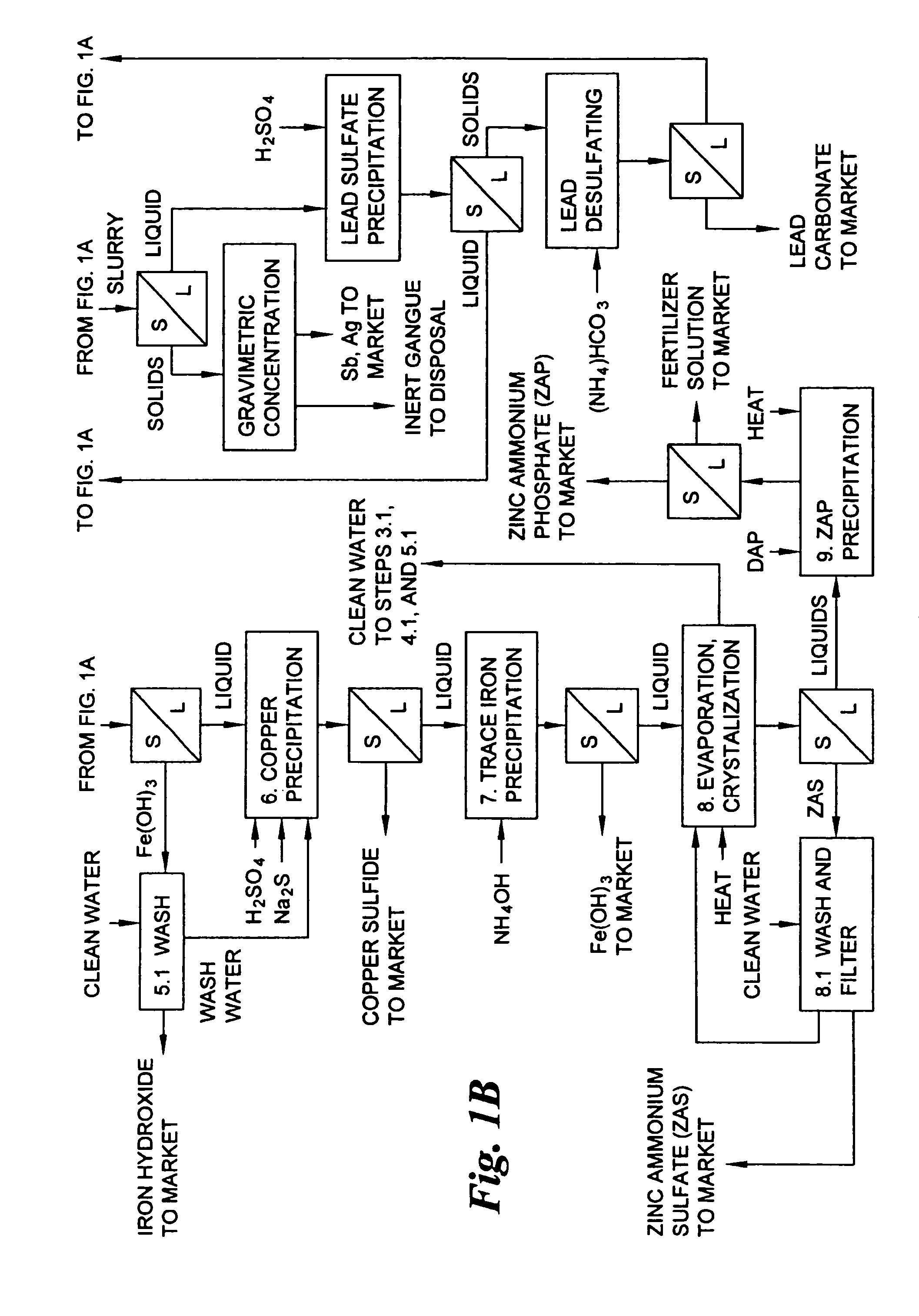
Hydrometallurgical process for the treatment of metal-bearing sulfide mineral concentrates
InactiveUS20070098609A1Improve efficiencyReduce the amount requiredSolvent extractionGold compoundsAmmonium compoundsMetallic sulfide
A hydrometallurgical process for the treatment of complex silver-bearing sulfide ores and concentrates that recovers substantially all silver, lead, antimony, zinc, copper and sulfur, along with the chemical reagents utilized during the process. Finely ground ores and concentrates are leached under heat and pressure with water, sulfuric acid, nitric acid, oxygen, and a catalyst, and are further treated to recover silver in the form of silver chloride; iron in the form of iron hydroxide; copper and all traces of soluble toxic metals as sulfides; zinc as zinc ammonium sulfate and specifically nitric acid, sulfuric acid, oxygen, ammonia, and ammonium compounds as valuable fertilizer products.
Owner:ROYAL SILVER PANAMA
Method for preparing modified micro granules
InactiveCN102139197AImprove targetingExcellent secondary particle sizeCalcium/strontium/barium carbonatesPigmenting treatmentBoiling pointAqueous solution
The invention discloses a method for preparing modified micro granules. Co-precipitation reaction is performed in aqueous solution and between the freezing point and the boiling point of reaction mother solution to generate the micro granules or mixed sediment of micro granule precursor and inorganic sediment. The problems of contradiction between granule micronization and surface modification and difficult separation of the micro granules and the reaction mother solution in the conventional method for preparing the modified micro granules are solved, and the brand-new industrialized production method for modified micro, submicron or nano granules is provided.
Owner:张颖
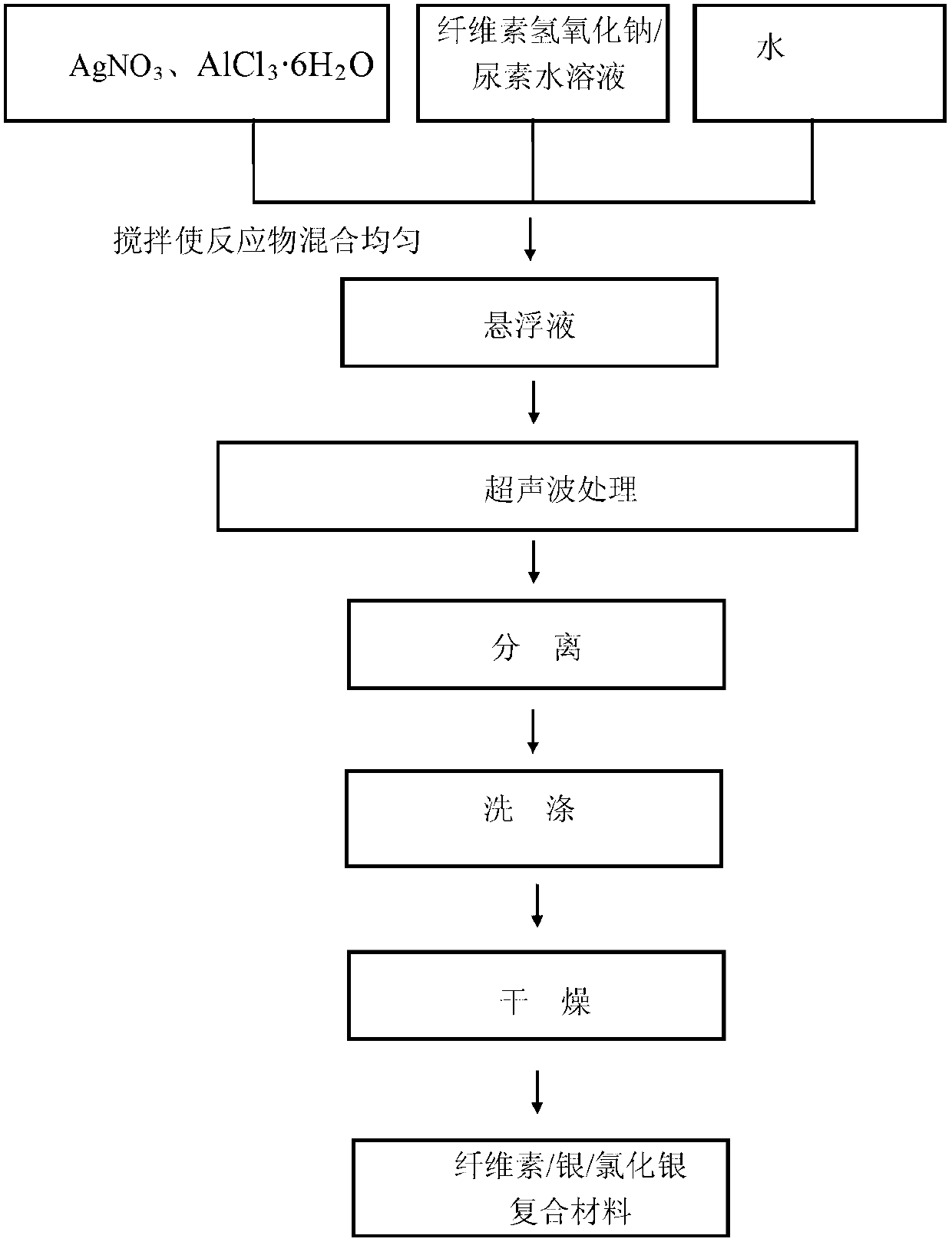
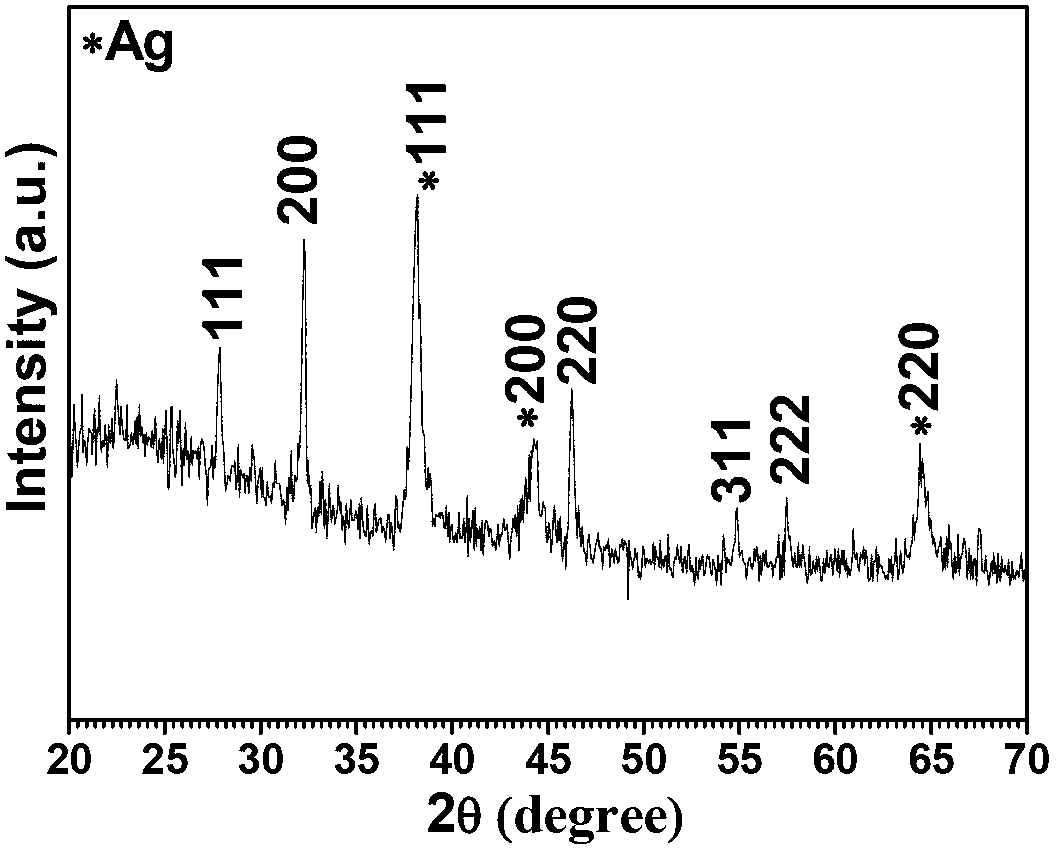
Cellulose/sliver/silver chloride composite material and preparation method thereof
The invention provides a cellulose / silver chloride composite material and a preparation method thereof. The cellulose / silver chloride composite material is quickly prepared by adopting microcrystalline cellulose, sliver salt and chlorine compound as the materials, adopting water as solvent and adopting an ultrasonic-wave treatment method. The cellulose / silver chloride composite material prepared by the preparation method disclosed by the invention is uniform in silver / silver chloride nanometer particle, capable of being uniformly distributed on a cellulose substrate and is extensive in application prospect in the antibacterial filed and the photocatalysis filed. Moreover, according to the preparation method disclosed by the invention, the materials are cheap, the process is simple, the operation is convenient, the preparation is quick and the process conditions are easy to control, so that the production process is greatly simplified, the time is saved and the cost is reduced. Meanwhile, the preparation method is free of any expensive and complex equipment and is beneficial to the industrial popularization.
Owner:BEIJING FORESTRY UNIVERSITY
Application of lactam as solvent in nano-grade material preparation
ActiveCN103204525AIncrease added valueMeet the requirements of green environmental protectionMaterial nanotechnologySilicaSolventMacromolecule
Disclosed is an application of lactam as a solvent in nanomaterial preparation. The preparation method comprises a precipitation method, a sol-gel method or a high-temperature pyrolysis method. The method achieves recycling utilization of the lactam solvent, which meets the requirements of environmental protection.
Owner:SHANGHAI GENIUS ADVANCED MATERIAL (GRP) CO LTD
Prepn process of nano silver iodide powder
InactiveCN1673093AHigh purityLow costNanostructure manufactureIodide preparationSilver iodideGranularity
The present invention relates to the preparation process of nanometer silver iodide powder. Clear silver sulfate water solution containing silver nitrate in 0.1-0.4 M / L and complexing agent in 0.034-0.2 M / L is dropped slowly into clear potassium iodide water solution containing potassium iodide in 0.1-0.4 M / L, complexing agent in 0.034-0.2 M / L and dispersant in 0.01-0.04 M / L at normal temperature and normal pressure via stirring, and the product is let stand to deposit to obtain nanometer silver iodide deposit. The nanometer silver iodide deposit is filtered, washed and dried at 80-100 deg.c for 1-2.5 hr to obtain nanometer silver iodide powder with granularity below 100 nm. The process is easy to control, pollution-free and suitable for industrial production, and the product has high quality and low cost.
Owner:JILIN UNIV
Method for manufacturing metal oxide hollow nanoparticles and metal oxide hollow nanoparticles manufactured by the same
ActiveUS20060269463A1Maintain good propertiesCopper oxides/halidesManganese oxides/hydroxidesNanoparticleOxygen
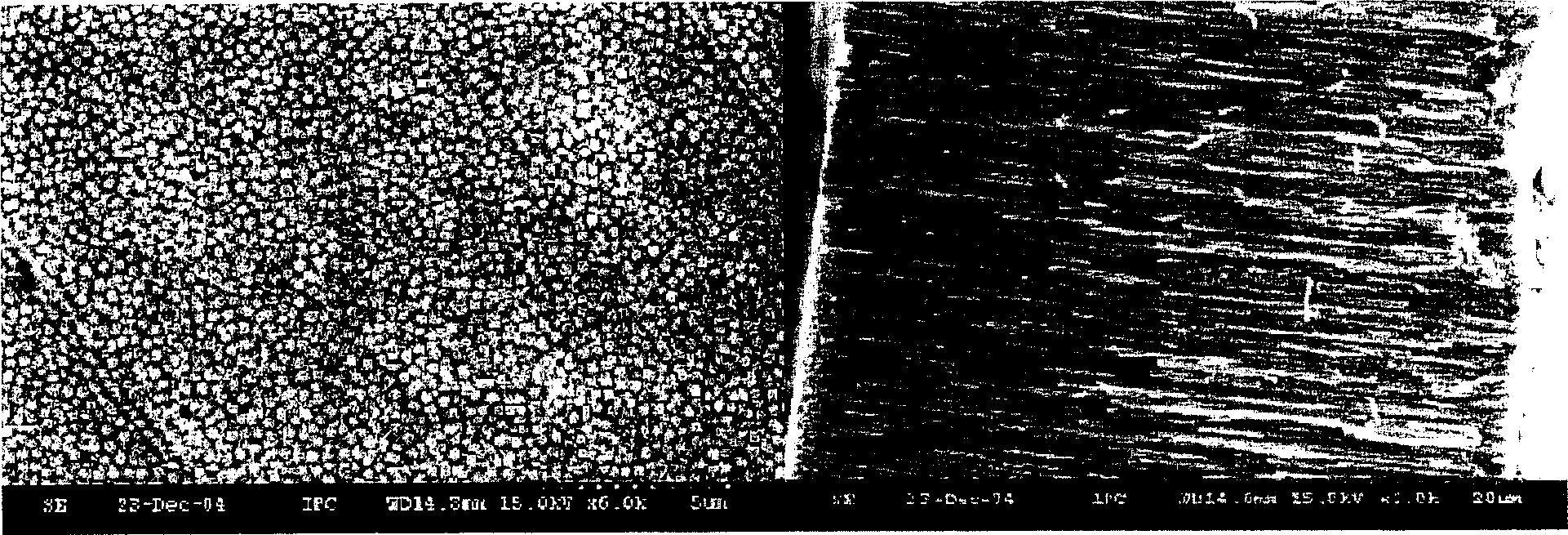
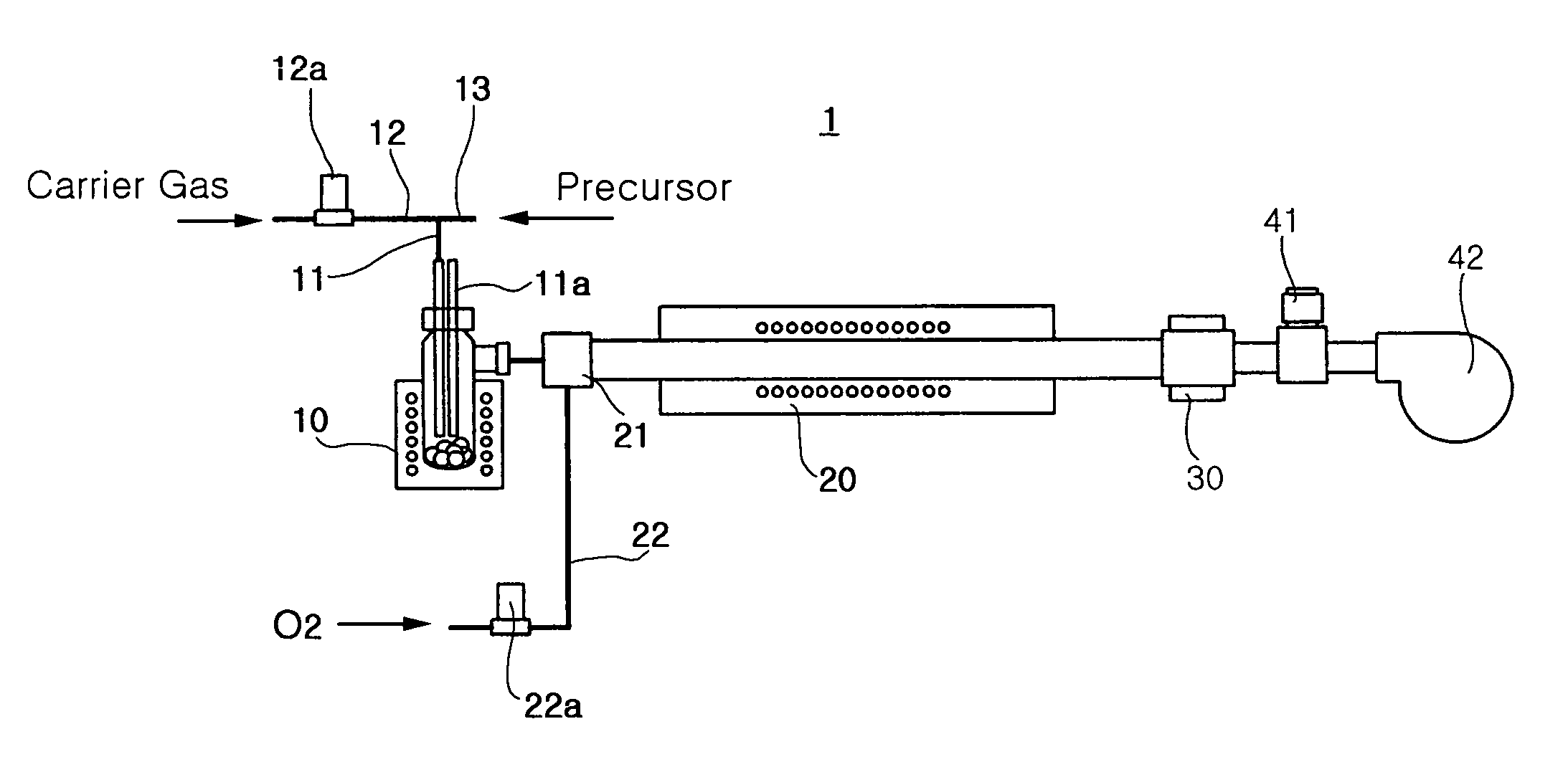
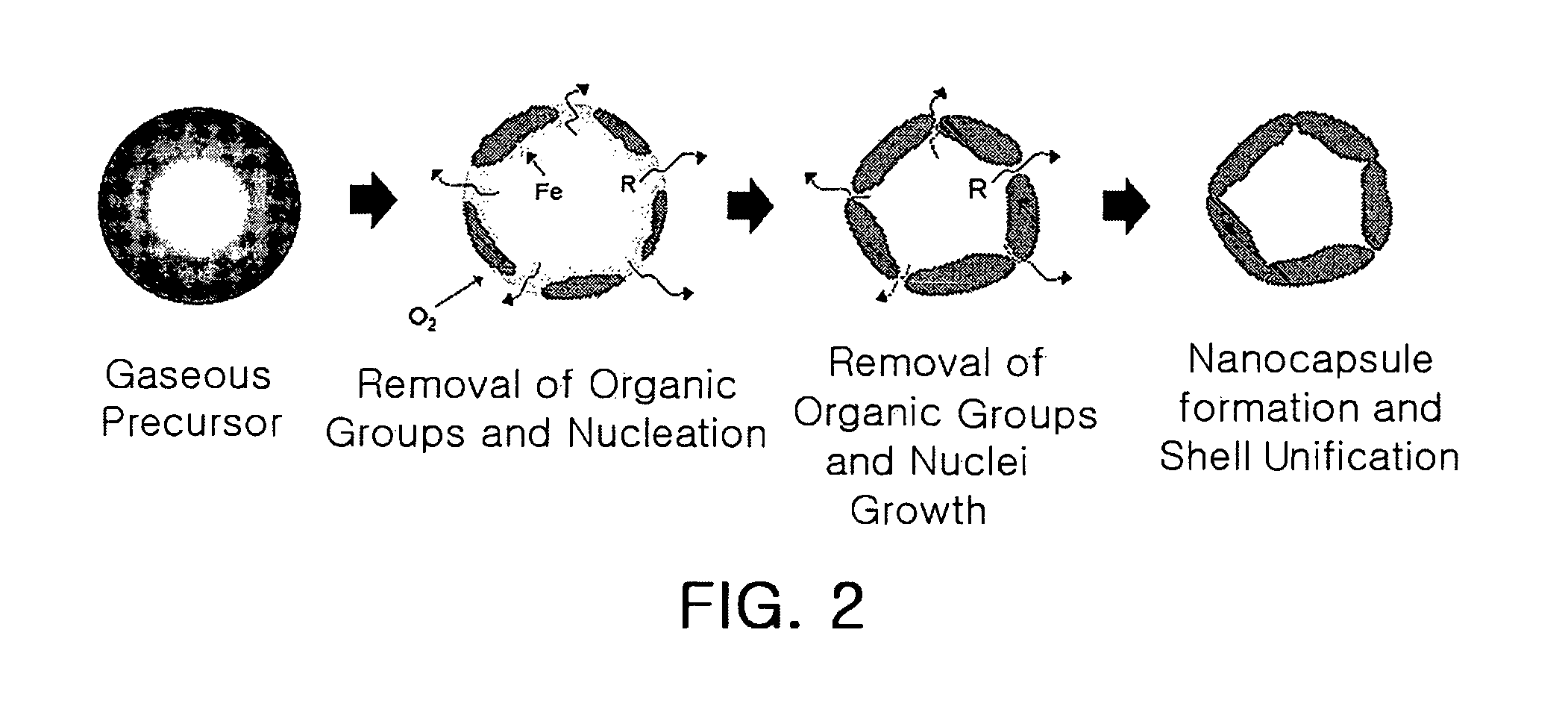
Provided is a method for manufacturing a metal oxide hollow nanoparticles with excellent properties more easily and simply by a chemical vapor condensation employing metal β-diketonates as precursors, and a metal oxide hollow nanoparticles manufactured by the method. The method includes: preparing metal β-diketonate as a precursor; evaporating the metal β-diketonate at a predetermined temperature higher than a melting point of the metal β-diketonate; transferring the evaporated metal β-diketonate into a reaction region; thermally decomposing the transferred gaseous metal β-diketonate and simultaneously inducing a reaction of the transferred gaseous metal β-diketonate with oxygen to synthesize the metal oxide hollow nanoparticle; and condensing and collecting the synthesized metal oxide hollow nanoparticles.
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV)
Method for preparing temperature-resistance nano AgCl SOL
InactiveCN1850613AImprove adsorption capacityEasy to washSilver halidesChlorideTemperature resistance
The invention discloses a method for preparing high temperature-resistant nano AgCl sol, comprising the steps of: a. preparing soluble chloride solution 0.01-0.2 mol / L, mixing it with dispersant 0.01-2 mol / L in the molar mass ratio of 1 to (1-4), and dispersing uniformly at a speed of 400-1000 r / min; b. heating the prepared solution to 25 deg.C-100 deg.C, regulating pH value of solution to 2-9, uniformly dropping in AgNO5 0.05-0.5mol / L and dispersant 0.01-2mol / L in the molar mass ratio of 1 to (0.25-10) at a speed of 0.01-0.1 ml / min, and dispersing uniformly at 1000-1500 r / min for 10-45 min, and processing the prepared nano sol with ultrasonic for 1 h. And its beneficial effect: at a certain temperature, the absorption of nano silver sol on fabric is high, and the nano silver sol is firm to absorb and uneasy to wash out.
Owner:DONGHUA UNIV
Process for preparing nano-grade AgCl powder by semisolid method
The invention relates to a method of preparing a nano-scale silver chloride powder by semi-solid-state method. The method comprises the steps of adopting AgNO3 and KCI as reactants, and adopting sulfonating succinate, polyethylene glycols or fatty alcohol polyethenoxy ether as dispersants, to respectively formulate a sulfonating succinate dispersant solution, a polyethylene glycol dispersant solution or a fatty alcohol polyethenoxy ether dispersant solution; adding any dispersant solution into the reactant KCI before the reaction of the reactant to obtain a reaction material with good moisture dispersion; adding AgNO3 powder to KCI with good moisture dispersion; adding the dispersant solution additionally according to the moistening degree of AgNO3, so as to cause AgNO3 to reach the moistening degree; conducing the in-situ semi-solid-state reaction process under grinding; conducting the procedures of in-situ purification of a product, the drying of the product and the grinding and the shaping of the product to obtain the silver chloride powder. By the method, the yield can be reach up to 91 percent to 95 percent. The prepared silver chloride powders are nano-sclae silver chloride powders which have regular spherical shapes, clear boundary lines among particles, high dispersion state, uniform distribution of particles and average fineness being 50-70nm.
Owner:NAVAL UNIV OF ENG PLA
Preparation method of water-soluble silver halide nanoparticle
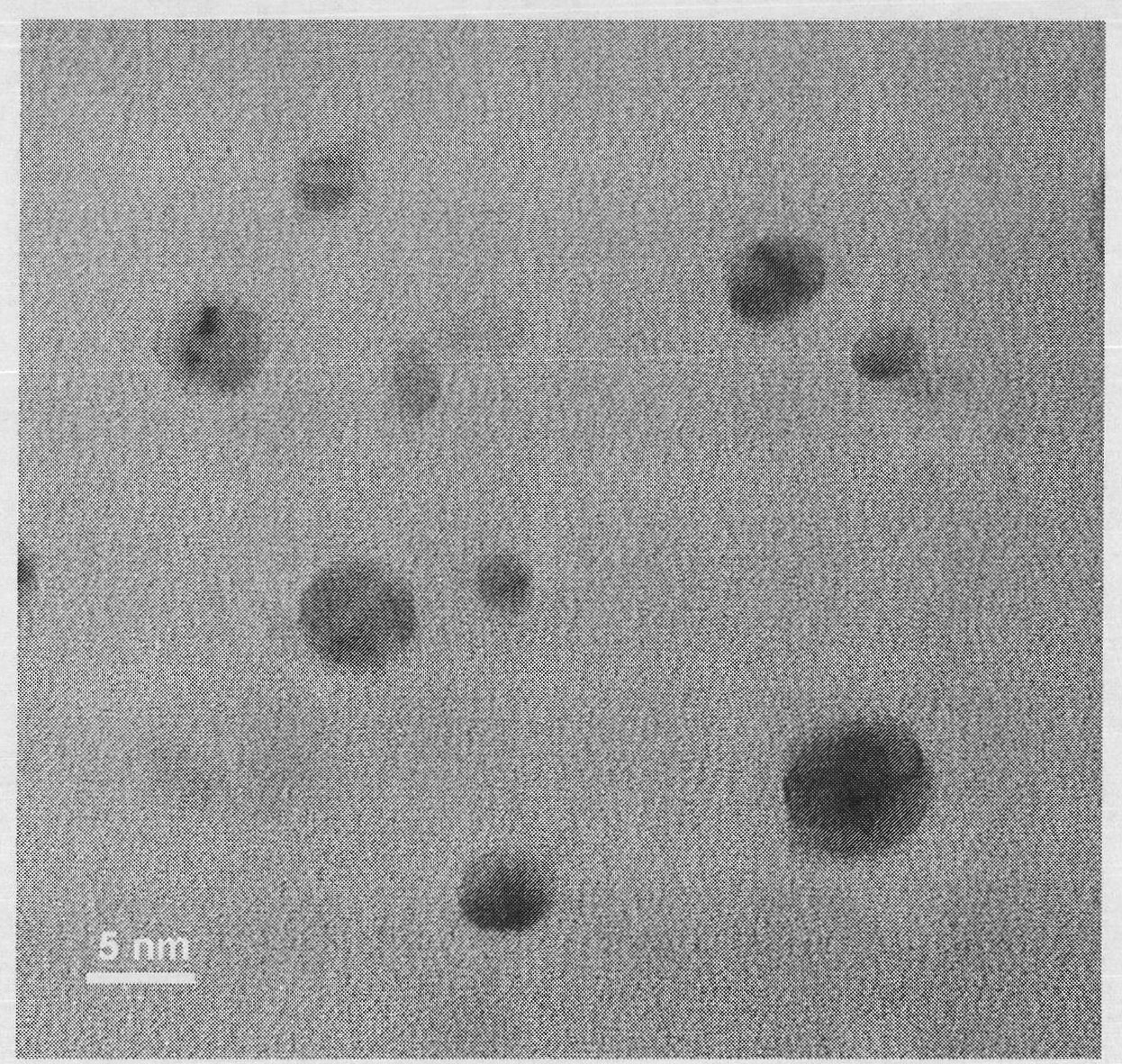
ActiveCN101774630AEasy to operateRaw materials are easy to getNanostructure manufactureSilver halidesSolubilityX-ray
The invention discloses a preparation method of a water-soluble silver halide nanoparticle, which comprises the following steps: adding 0.1-50g of hyperbranched polyglycidol with the molecular weight being 10000-500000 into 50-1000mL of deionized water, stirring for 1 minute to 1 hour, adding 0.01-5g of silver salt, continuously stirring for 1 minute to 2 hours, adding halogen-containing acid or salt with the molar ratio of the halogen-containing acid or salt and the silver salt being 0.43-1.05, and stirring 1 minute to 1 hour, thereby obtaining the water-soluble silver halide nanoparticle. The particle size of the water-soluble silver halide nanoparticle is 2-20nm, the size distribution is very uniform, and the water solubility is good. The operation of the invention is simple, the raw materials can be easily obtained, and the invention is applicable to large-scale preparation. The particle size of the water-soluble silver halide particle prepared in the invention is observed by a transmission electron microscope, the element composition of the water-soluble silver halide particle is tested by an energy spectrum analyzer, and the crystal structure of the water-soluble silver halide particle is represented by an X-ray diffraction instrument. The results show that the particle size of the prepared water-soluble silver halide nanoparticle is 2-20nm, and the crystallization is good.
Owner:ZHEJIANG TANGUSHANGXI MATERIAL SCI & TECH
Silver Iodate Compounds Having Antimicrobial Properties
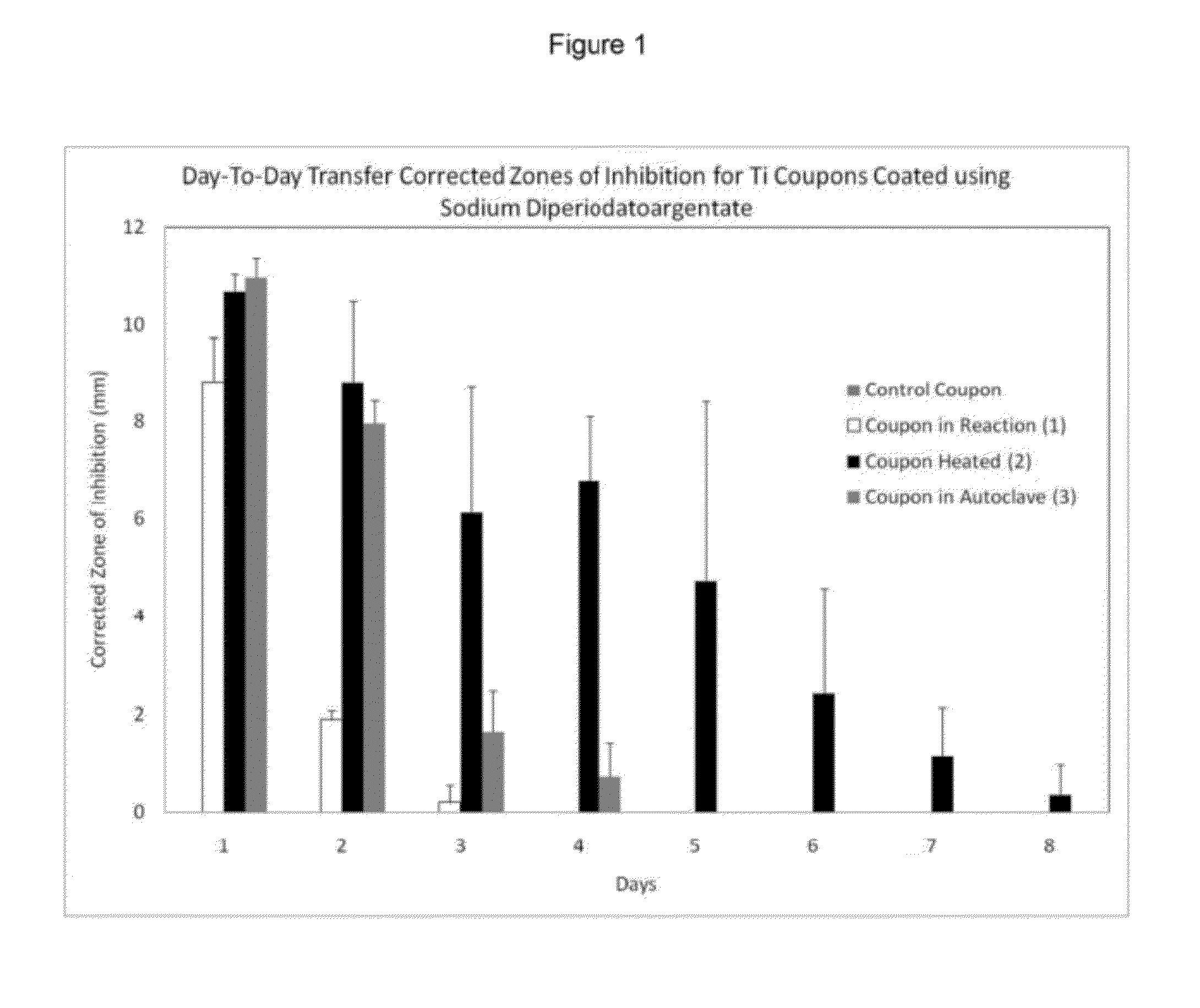
The present invention is compositions, methods of use, methods of treating, and articles of manufacture that include at least one silver iodate for imparting antimicrobial properties, particularly as it relates to the manufacture, use, and properties of medical devices. The invention also includes obtaining and using one or more silver iodate reaction products from a diperiodatoargentate, wherein the reaction products are obtained using a hydrothermal reaction.
Owner:OLSON MERLE E +3
Anti-virus aluminum member and method for producing same
ActiveCN103781945AIncreased durabilityLong-term antiviralAnodisationAntiviralsAnti virusSecondary Infections
The present invention provides an anti-virus aluminum member capable of minimizing secondary infection by deactivating viruses in a short period of time even when viruses adhere thereto, regardless of whether a viral envelope is present, and useful for application in door knobs, handrails, air-conditioner fins or the like. An anti-virus aluminum member capable of deactivating viruses that adhere thereto is characterized in that an anti-virus inorganic compound is present in the pores of an anodized membrane provided with multiple pores and obtained by anodizing aluminum or an aluminum alloy.
Owner:NBC MESHTEC
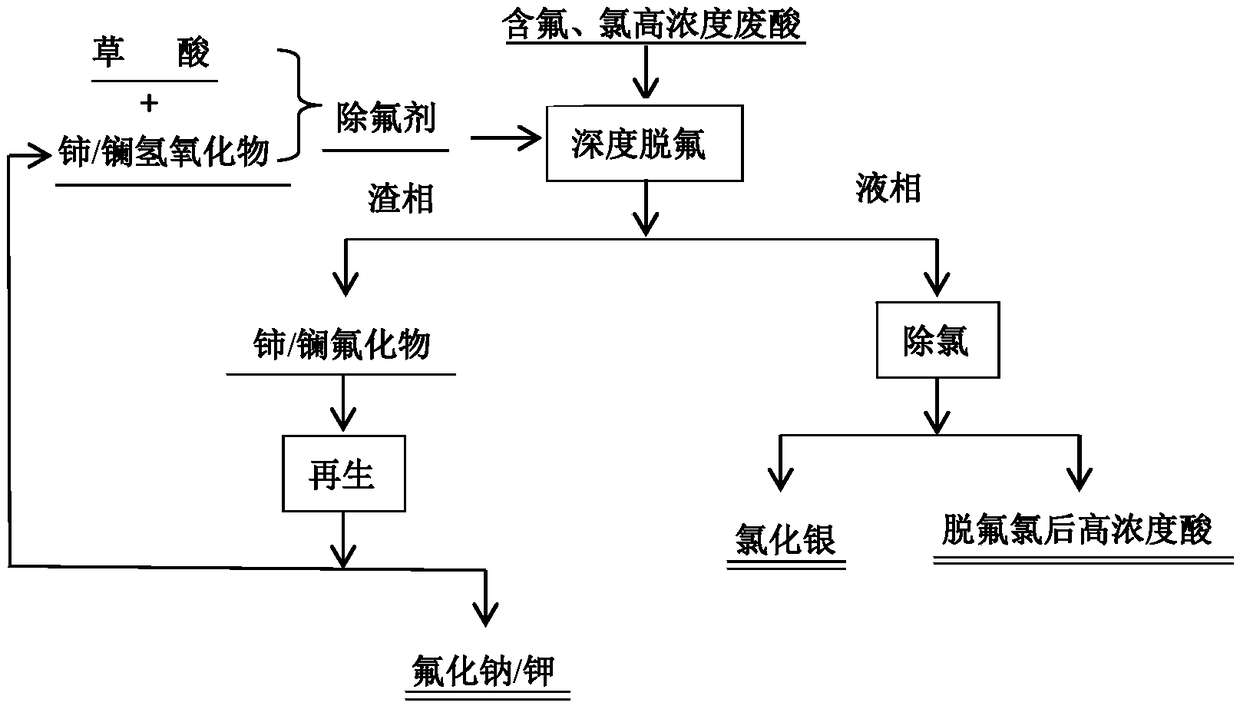
Method for removing fluorine and chloride ions from high-concentration industrial waste acid
ActiveCN108642503ASimple processReduce investmentSulfur-trioxide/sulfuric-acidNitric acidHigh concentrationSlag
The invention provides a method for removing fluorine and chloride ions from high-concentration industrial waste acid and belongs to the technical field of high-concentration industrial waste acid treatment. The method comprises the steps that a rare earth hydroxide solution and a fluorine removal agent of oxalic acid are added to the high-concentration industrial waste acid to carry out defluorination treatment to obtain a slag phase and a liquid phase; then a slag phase alkali solution obtained after the defluorination treatment is carried out is separated to obtain easily-dissolved fluorideand rare earth hydroxide precipitation, and the rare earth hydroxide precipitation and the oxalic acid serve as the fluorine removal agent to carry out new-round defluorination treatment; and meanwhile, a silver nitrate solution is added to the liquid phase obtained after the defluorination treatment to obtain silver chloride precipitation and high-concentration acid, and the high-concentration acid is used in the stainless steel acid pickling working procedure. According to the method, the fluorine and chloride ions can be removed from the high-concentration industrial waste acid, the fluorine removal agent can be recycled, resources are saved, and the requirement for environmental protection of clean production is met.
Owner:UNIV OF SCI & TECH BEIJING
Process for chlorinating resources containing recoverable metals
A process for chlorinating ore, slag, mill scale, scrap, dust and other resources containing recoverable metals from the groups 4-6, 8-12, and 14 in the periodic table. The process comprises: a) forming a liquid fused salt melt consisting essentially of aluminum chloride and at least one other metal chloride selected from the group consisting of alkali metal chlorides and alkaline earth metal chlorides, wherein the aluminum chloride content in the liquid salt melt exceeds 10% by weight; b) introducing the recoverable metal resources into said liquid salt melt: c) reacting the aluminum chloride as chlorine donor with said recoverable metal resource to form metal chlorides, which are dissolved in the salt melt; and d) recovering the formed metal chlorides from the salt melt.
Owner:P M TECH
Chemical deposition process of preparing composite nanometer mesoporous silver halide/alumina material
InactiveCN1847139ANanostructure manufactureSurface reaction electrolytic coatingMesoporous materialSilver ion
The present invention belongs to the field of nanometer mesoporous material technology, and is especially chemical deposition process of preparing composite nanometer mesoporous silver halide / alumina material with porous alumina template as mesoporous solid. The preparation process includes the first ultrasonic treatment, and soaking in gelatin solution, hardening agent solution and halogen anion salt solution successively of AAO template; the subsequent reaction of the treated template in a connector in the shown structure and with silver ion solution and halogen and silver ion solution filled separately on two sides of the template; and final soaking the reaction product in water solution of glycerin to eliminate gelatin and in deionized water to eliminate impurity ions. The present invention has the pores in AAO template filled with nanometer AgX lines in the length comparative with the thickness of AAO template.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for manufacturing metal oxide hollow nanoparticles and metal oxide hollow nanoparticles manufactured by the same
ActiveUS8052958B2Maintain good propertiesCopper oxides/halidesManganese oxides/hydroxidesNanoparticleGas phase
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV)
Park floor lamp capable of automatically dissipating heat and expelling insects
InactiveCN111550702ANo input requiredAid in diffusionLighting heating/cooling arrangementsProtective devices for lightingCold airStructural engineering
The invention discloses a park floor lamp capable of automatically dissipating heat and expelling insects. The lamp comprises a lamp shell and a lamp tube installed in the lamp shell, an installationgroove is formed in the lamp shell, the installation groove is located above the lamp tube, a light-transmitting plate is connected to the groove opening of the installation groove in a sealed mode, an equipment groove is formed in the light-transmitting plate, and a plurality of shading plates are symmetrically and rotationally installed in the equipment groove through a rotating shaft. The decomposition characteristic of the driving agent is utilized, when the lamp tube works, the heat dissipation fan can be autonomously driven to periodically rotate forwards and backwards, so that hot air in the shell is intermittently exhausted, and external cold air is sucked in, the flowing speed of gas in the shell and outside gas is increased, the heat dissipation effect is good, energy conservation and environmental protection are achieved, meanwhile, with forward rotation of the heat dissipation fan, the atomization spray head can spray stored insect expelling liquid to the ventilation groove, insects can be prevented from being gathered in the ventilation groove, and normal use of the heat dissipation opening is guaranteed.
Owner:侯儒仪
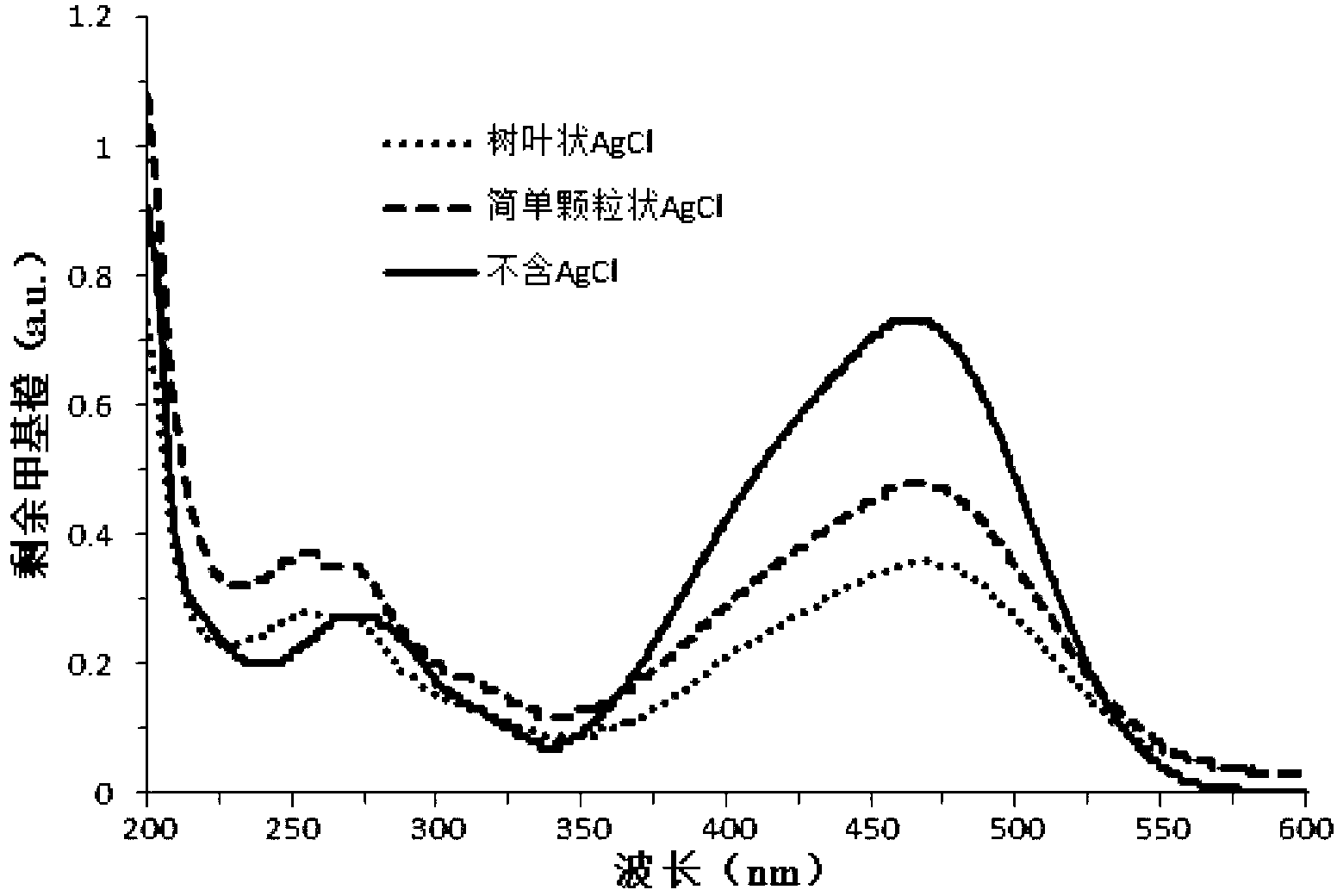
Preparation method for leaf-shaped silver chloride particle with micron structure
ActiveCN102701266AImprove stabilityImprove photocatalytic performancePhysical/chemical process catalystsSilver halidesCentrifugationHydrothermal synthesis
The invention relates to the technical field of hydrothermal synthesis of photocatalytic materials, in particular to a preparation method for a leaf-shaped silver chloride particle with a micron structure. The preparation method for the leaf-shaped silver chloride particle with the micron structure comprises the following steps: through hydrothermal reduction, mixing EG solutions of AgNO3, FeCl3 and PVP, which contain concentrated HCl; performing reaction for a certain time, and then cooling the solution; extracting a sample through centrifugation, and finally obtaining the high-content and axially-symmetrical leaf-shaped AgCl particle with the micron structure. The preparation method for the leaf-shaped silver chloride particle with the micron structure is simple in step and easy and convenient to operate; a larger proportion of concentrated HCl is added, the product is in a form of AgCl crystals and people usually use an Ag elementary substance obtained from the reaction composition and the preparation method; the scanning electron microscopy shows that the structure of the AgCl crystal particle with the micron structure has an unified axially-symmetrical triangular leaf shape; and a performance test shows that the leaf-shaped silver chloride particle with the micron structure has a good performance for photocatalytically degrading organic contaminants.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Hydrometallurgical process for the treatment of metal-bearing sulfide mineral concentrates
InactiveUS7892505B2Improve efficiencyReduce the amount requiredSolvent extractionGold compoundsAmmonium compoundsHydrometallurgy
Owner:ROYAL SILVER PANAMA
Method of preparing spectrally-pure superfine silver chloride
The invention relates to a method of preparing spectrally-pure superfine silver chloride, belonging to the technical field of inorganic chemistry. In order to solve a technical problem, the invention provides a method of preparing spectrally-pure superfine silver chloride. The method of preparing spectrally-pure superfine silver chloride comprises the following steps: a, getting analytically-pure silver nitrate liquor and then dropwise adding mixed liquor of hydrochloric acid, alcohol and ethyl ether into the liquor until no white precipitates are generated; b, standing, so that the precipitates are completely settled and filtered to obtain silver chloride precipitates; c, washing by adopting 40wt%-50wt% alcohol liquor; precipitating the silver chloride precipitates prepared in the step b for 2-4 times, and then, washing the silver chloride precipitates by several times by using distilled water of 50 DEG C-60 DEG C until pH value of the filtrate is 4.5-6.0 and no chlorine ions are detected in the filtrate by using silver nitrate; and d, drying the washed silver chloride precipitates to obtain spectrally-pure superfine silver chloride.
Owner:CDGM OPTICAL GLASS
Method of separation/purification for high-purity silver chloride and process for producing high-purity silver by the same
ActiveCN1795146AHigh purityEffective separation and refinementProcess efficiency improvementSilve compoundsElectrolysisSulfite
The present invention provides that when separating and refining high-purity silver chloride from refining intermediates, the aforementioned refining intermediates do not require pretreatment and when using them as raw materials to obtain metallic silver, dry refining of metallic silver or reprocessing based on electrolysis may not be performed. Purification treatment, to obtain high-purity silver high-purity silver chloride effective separation and purification method, and the use of the high-purity silver chloride to manufacture high-purity silver method. It is characterized in that: comprising, leaching the aforementioned refining intermediate in an aqueous sulfite solution, extracting silver into the solution to form a leaching solution containing silver and an insoluble residue; neutralizing the aforementioned leaching solution into an acidic , to precipitate silver chloride, to form the silver chloride and the silver chloride generation process of the mother liquor; Silver chloride refining process for solutions containing impurity elements.
Owner:SUMITOMO METAL MINING CO LTD
Method for preparing silver chloride sol and silver sol from AgCl powder
InactiveCN101475205ASimplify operating proceduresEasy to manufactureSilver halidesSilver chlorideSolvent
The invention discloses a method for preparing silver chloride sol and silver sol from AgCl powder, which comprises the following two steps that: (1) AgCl powder is dissolved in a mixed solution of DMF and HCl, namely the formation of an Ag-Cl complex solution; and (2) the Ag-Cl complex solution reacts with a solvent (one of water, methanol and ethanol or a mixed solvent), AgCl sol can be obtained under the control of PVP, the pH value of the Ag-Cl complex solution is adjusted to be between 10.0 and 11.5, and yellow silver nano-sol can be obtained under heating conditions. The method has the advantages of simple process, easy realization, and lower cost.
Owner:EAST CHINA UNIV OF SCI & TECH
Solid non-polarized electrode resistant to deep sea pressure and preparation method thereof
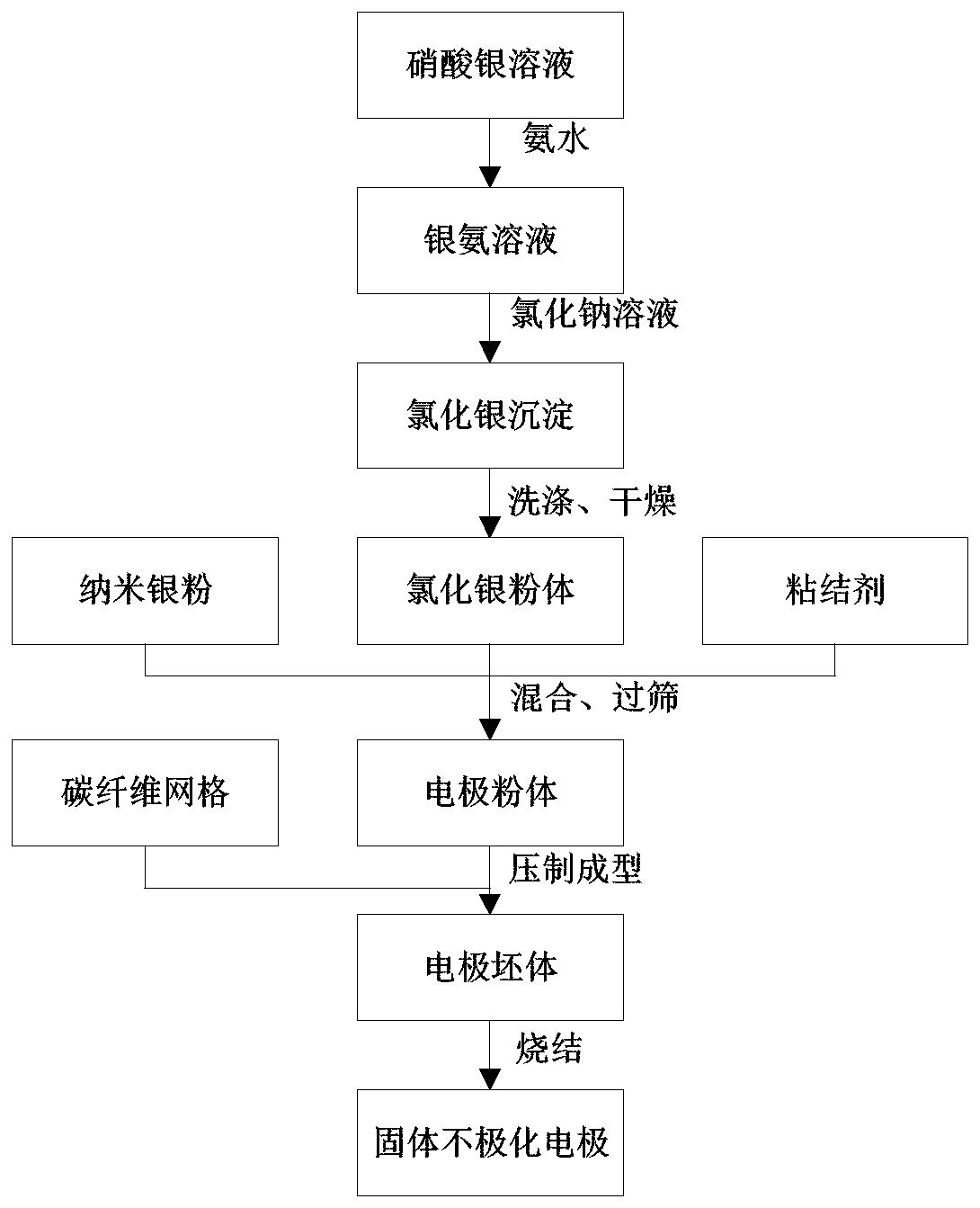
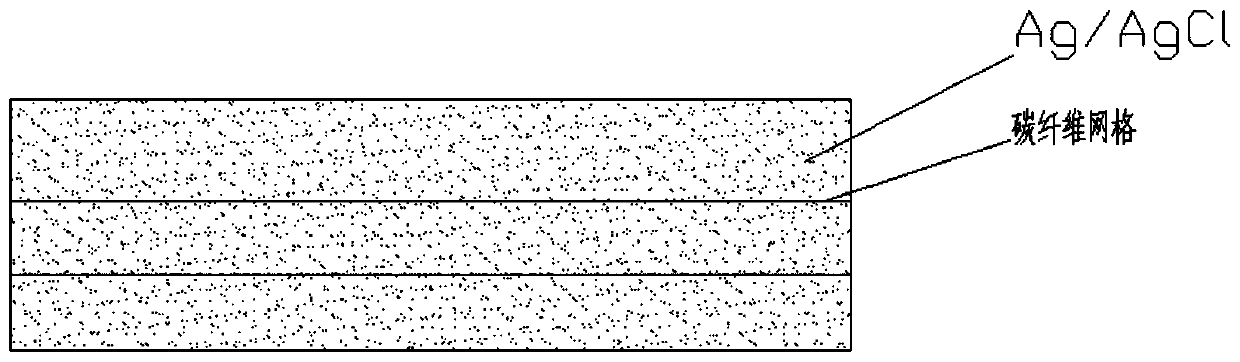
ActiveCN110045428AExtremely smallPrevent oxidationTransportation and packagingMetal-working apparatusFiberCarbon fibers
The invention provides a preparation method of a solid non-polarized electrode resistant to a deep sea pressure. The preparation method of the solid non-polarized electrode resistant to the deep sea pressure comprises the following steps: preparing a silver ammonia solution by using AgNO<3> and ammonia water, and adding a NaCl solution to the silver ammonia solution to obtain a silver chloride precipitate; washing the silver chloride precipitate clean to obtain a AgCl powder after drying; mixing the AgCl powder with a nano silver powder and a binder and screening to obtain an electrode powder;and pressing the screened electrode powder under a certain pressure for molding; and in the molding process, carbon fibers woven into a grid shape are added to the electrode powder, and the molded green body is sintered under a gas atmosphere, so that the solid non-polarized electrode is obtained. The preparation method provided by the invention is simple; and the solid non-polarized electrode prepared by the preparation method provided by the invention has high mechanical strength, can withstand deep sea pressure, and has small electrode difference and stable performance, which can be used for detecting electric field signals in deep sea environment.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Silver halide based photoinitiator and application thereof to light-induction unsaturated olefin monomer polymerization
InactiveCN103804549AQuick responseMild reaction conditionsSilver halidesUltraviolet lightsSalt water
The invention belongs to the technical field of photo-initiation polymer polymerization and particularly relates to a silver halide based photoinitiator and application of the silver halide based photoinitiator to light-induction unsaturated olefin monomer polymerization. Active silver halide based material is used as a photoinitiator, and organic polymer material is prepared through induction polymerization under ultraviolet light. Firstly, surface active agent is added into halide salt water solution, then silver nitrate solution is added under a stirring condition to perform reaction, and the active silver halide based photoinitiator is obtained through dialysis, the silver halide based photoinitiator, the unsaturated olefin monomer and water are mixed to form stable dispersion liquid, active silver halide particles in the silver halide based photoinitiator absorb ultraviolet light to generate free radical under the irradiation of an ultraviolet lamp, unsaturated olefin monomer polymerization is initiated, and then the organic polymer material is prepared. The polymerization reaction can be carried out in water phase, the using range of suitable monomers and low polymers is wide, and the silver halide based photoinitiator has the characteristics of quick reaction speed, gentle reaction condition, environment protection and the like.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
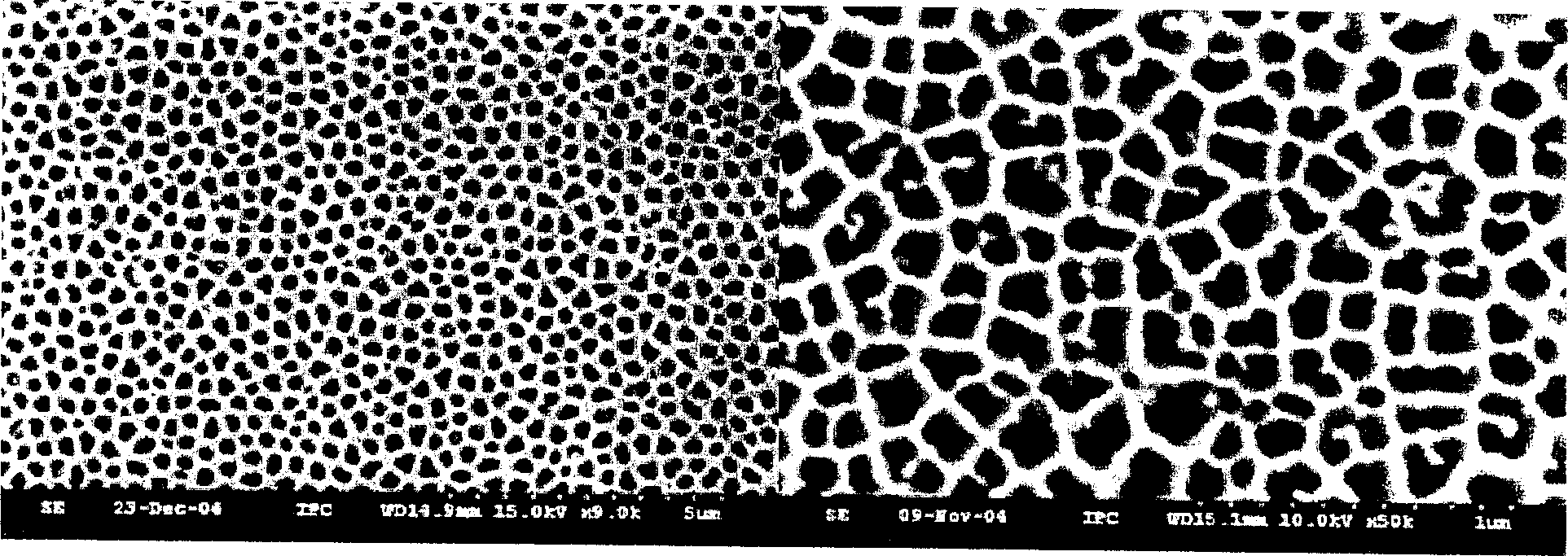
Three-dimensional dendritic silver chloride crystal growing in direction (111) and preparation method thereof, and photocatalyst
InactiveCN102795656AAdjustable sizeAdjustable lengthPhysical/chemical process catalystsSilver halidesSynthesis methodsRoom temperature
The invention discloses a high-activity three-dimensional dendritic silver chloride crystal growing in direction (111) and a preparation method of the high-activity three-dimensional dendritic silver chloride crystal. The crystal has a three-dimensional dendritic structure; and the three-dimensional dendritic silver chloride crystal is obtained through the following steps of: dissolving an oxidant in de-ionized water and thoroughly stirring for 2 minutes at the room temperature; adding sodium chloride to the stirred solution and evenly mixing the mixture by stirring for 2 minutes; next, adding citric acid to the mixed solution, stirring for 2 minutes, and then putting a silver slide into the solution for reaction at the room temperature for 2-4 hours, thereby obtaining the target product. The invention also provides a photocatalyst prepared from the silver chloride crystals under illumination. The preparation method of the high-activity three-dimensional dendritic silver chloride crystal is simple in synthesis method and steps; the product is good in uniformity and suitable for large-scale production; the synthesized three-dimensional dendritic silver chloride crystal has surfaces formed by high-index crystal faces, and is high in surface activity; and the photocatalyst formed from the silver chloride crystals under illumination has strong catalytic activity.
Owner:SHANDONG UNIV
Preparation method of rod-shaped AgBr nanometer material
InactiveCN103172106AReduce yieldEasy to useMaterial nanotechnologySilver halidesNew energyInorganic compound
The invention discloses a preparation method of a rod-shaped AgBr nanometer material, and belongs to the technical field of preparation of inorganic compound new-energy materials. According to the preparation method, the rod-shaped AgBr nanometer material is prepared by adopting a hydrothermal method and taking sodium bromide, silver acetate, dimethyl sulfoxide and polyvinyl pyrrolidone as raw materials. The preparation method particularly comprises the following steps of: dissolving the polyvinyl pyrrolidone and the sodium bromide into a dimethyl sulfoxide / deionized water mixed solvent at room temperature, and then slowly dropping a silver acetate water solution to generate a precursor; carrying out hydrothermal reaction in a reaction kettle at 130 DEG C for 2-14 hours; and after the hydrothermal reaction is finished, centrifugalizing, washing by using deionized water and anhydrous ethanol for multiple times, and drying to obtain the rod-shaped AgBr nanometer material. The preparation method disclosed by the invention has the advantages of easiness for operation, low raw material price, high yield, and the like.
Owner:BEIHANG UNIV
High-activity cubic-block silver chloride micron-crystal and electrochemical preparation method thereof
InactiveCN103466684AEasy to adjust the sizeImprove uniformityPhysical/chemical process catalystsElectrolysis componentsElectrochemical responseThermostat
The invention relates to a high-activity cubic-block silver chloride micron-crystal and an electrochemical preparation method thereof. The high-activity cubic-block silver chloride micron-crystal is of pure cubic-phase silver chloride and has a {100}-face-exposed crystal face structure. The method comprises the steps of adding sodium sulfate into a certain volume of deionized water, stirring to dissolve solids, then, adding sodium chloride, and stirring for mixing uniformly; transferring the prepared mixed solution into an H-shaped electrochemical reaction tank, adding a silver sheet to one end of the reaction tank, connecting the end of the reaction tank to the positive pole of a constant-potential power supply, adding a platinum sheet to the other end of the reaction tank, and connecting the other end of the reaction tank to the negative pole of the constant-potential power supply; regulating the voltage output by the power supply, oxidizing the silver sheet so as to generate silver ions, reacting for 30-40 minutes at room temperature, filtering and washing precipitates at the bottom of the reaction tank nearby the silver sheet electrode, and drying in a thermostat, thereby obtaining the cubic-block silver chloride micron-crystal. Under visible light, the activity of a photocatalyst formed by the cubic-block silver chloride micron-crystal is relatively high.
Owner:SHANDONG UNIV
Method for recovering silver ions from tantalum electrolytic capacitor by using novel chelate resin
InactiveCN105413651AWide variety of sourcesHigh mechanical strengthOther chemical processesProcess efficiency improvementN dimethylformamideElectrolysis
The invention discloses a method for recovering silver ions from a tantalum electrolytic capacitor by using novel chelate resin. A preparation method for the chelate resin comprises the following steps: (1) soaking chlorine spheres in N,N-dimethylformamide, which serves as a reaction solvent, until the chlorine spheres are thoroughly swollen; (2) adding 2-amino-4-methylpyridine, which serves as a ligand, and sodium, which serves as a catalyst, into a product of the step (1), and carrying out stirred reaction for 10 hours at the temperature of 80 DEG C under nitrogen protection, wherein the mass ratio of 2-amino-4-methylpyridine to -CH2Cl in the chlorine spheres is 4: 1; and (3) filtering a product of the step (2) so as to obtain a filter cake, firstly, washing the filter cake with the reaction solvent by soaking until a washing solution is colorless, then, washing the filter cake with distilled water, then, repeatedly washing the filter cake with anhydrous ethanol, acetone, diethyl ether and distilled water sequentially, and carrying out vacuum drying on the filter cake at the temperature of 50 DEG C, thereby obtaining the novel chelate resin. The method has the characteristics that the novel chelate resin selectively adsorbs the silver ions in the tantalum electrolytic capacitor, is good in adsorption performance, can be reused and the like.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Preparation method of rod-shaped agbr nanomaterial
InactiveCN103172106BReduce yieldEasy to useMaterial nanotechnologySilver halidesNew energyInorganic compound
The invention discloses a preparation method of a rod-shaped AgBr nanometer material, and belongs to the technical field of preparation of inorganic compound new-energy materials. According to the preparation method, the rod-shaped AgBr nanometer material is prepared by adopting a hydrothermal method and taking sodium bromide, silver acetate, dimethyl sulfoxide and polyvinyl pyrrolidone as raw materials. The preparation method particularly comprises the following steps of: dissolving the polyvinyl pyrrolidone and the sodium bromide into a dimethyl sulfoxide / deionized water mixed solvent at room temperature, and then slowly dropping a silver acetate water solution to generate a precursor; carrying out hydrothermal reaction in a reaction kettle at 130 DEG C for 2-14 hours; and after the hydrothermal reaction is finished, centrifugalizing, washing by using deionized water and anhydrous ethanol for multiple times, and drying to obtain the rod-shaped AgBr nanometer material. The preparation method disclosed by the invention has the advantages of easiness for operation, low raw material price, high yield, and the like.
Owner:BEIHANG UNIV
Prepn process of nano silver iodide powder
InactiveCN1280200CHigh purityLow costNanostructure manufactureIodide preparationSilver iodideRoom temperature
Owner:JILIN UNIV
Method for preparing temperature-resistance nano AgCl SOL
InactiveCN100427402CImprove adsorption capacityNot easy to washSilver halidesChlorideTemperature resistance
The invention discloses a method for preparing high temperature-resistant nano AgCl sol, comprising the steps of: a. preparing soluble chloride solution 0.01-0.2 mol / L, mixing it with dispersant 0.01-2 mol / L in the molar mass ratio of 1 to (1-4), and dispersing uniformly at a speed of 400-1000 r / min; b. heating the prepared solution to 25 deg.C-100 deg.C, regulating pH value of solution to 2-9, uniformly dropping in AgNO5 0.05-0.5mol / L and dispersant 0.01-2mol / L in the molar mass ratio of 1 to (0.25-10) at a speed of 0.01-0.1 ml / min, and dispersing uniformly at 1000-1500 r / min for 10-45 min, and processing the prepared nano sol with ultrasonic for 1 h. And its beneficial effect: at a certain temperature, the absorption of nano silver sol on fabric is high, and the nano silver sol is firm to absorb and uneasy to wash out.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com