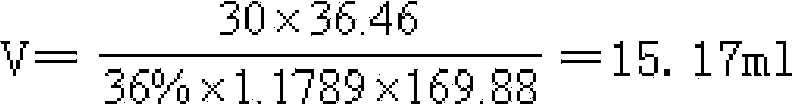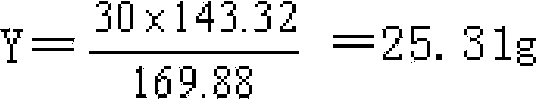Method of preparing spectrally-pure superfine silver chloride
A silver chloride and spectrally pure technology, applied in the direction of silver halide, etc., can solve the problems that the samples to be tested cannot be mixed uniformly, difficult to purchase, and cannot be ground into powder, so as to achieve effective analysis and testing, shorten the synthesis cycle, and improve The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The method for preparing spectroscopically pure ultrafine silver chloride of the present invention comprises the steps:
[0019] a, get analytically pure silver nitrate solution, then drop the mixed solution of hydrochloric acid, ethanol, diethyl ether mixed solution in the solution until no white precipitate is generated; wherein, the mixed solution adopts 36wt% hydrochloric acid solution, 95wt% ethanol solution, diethyl ether It is prepared by mixing according to the volume ratio of 3~5:2.5~3.5:0~1.2;
[0020] B, leave standstill, make precipitation settle completely, filter, obtain silver chloride precipitation;
[0021] c, adopt 40~50wt% ethanol solution to wash the silver chloride precipitate prepared in step b for 2~4 times, then wash the silver chloride precipitate several times with 50~60°C distilled water until the pH value of the filtrate is 4.5~6.0, and Until there is no chloride ion in the filtrate with silver nitrate detection;
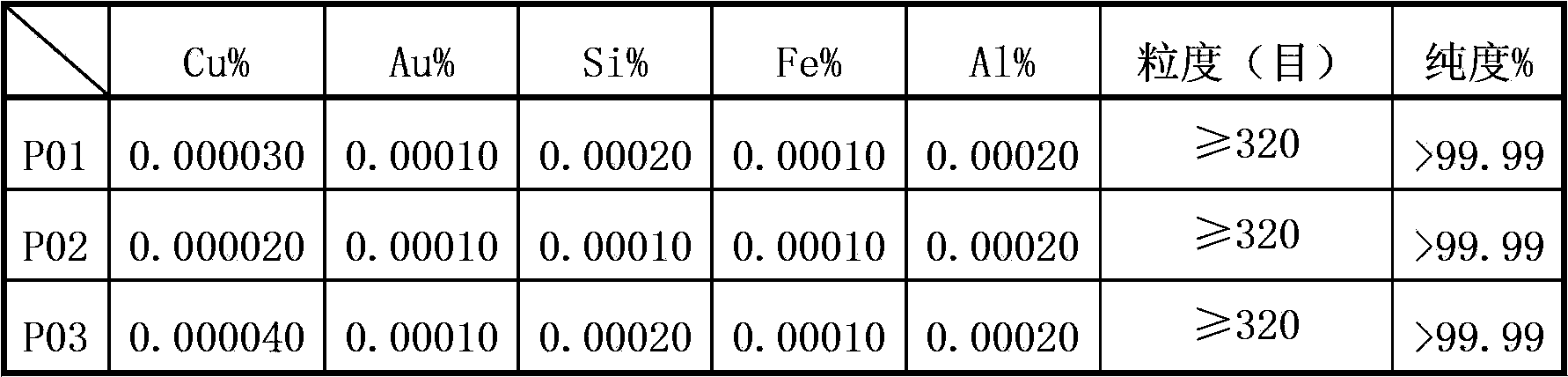
[0022] d. Dry the precipi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com