Preparation method of water-soluble silver halide nanoparticle
A nanoparticle and silver halide technology, applied in the field of nanomaterials, can solve problems such as difficult to obtain water-soluble silver halide nanoparticles, and achieve the effect of simple operation and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Step 1): Add 1g of hyperbranched polyglycidol with a molecular weight of 20000 to a flask containing 100mL of deionized water, and after stirring for 30min, add 0.2g of silver nitrate and continue stirring for 1hr to obtain a hyperbranched polyglycidol containing silver ions. Aqueous glycidol solution.
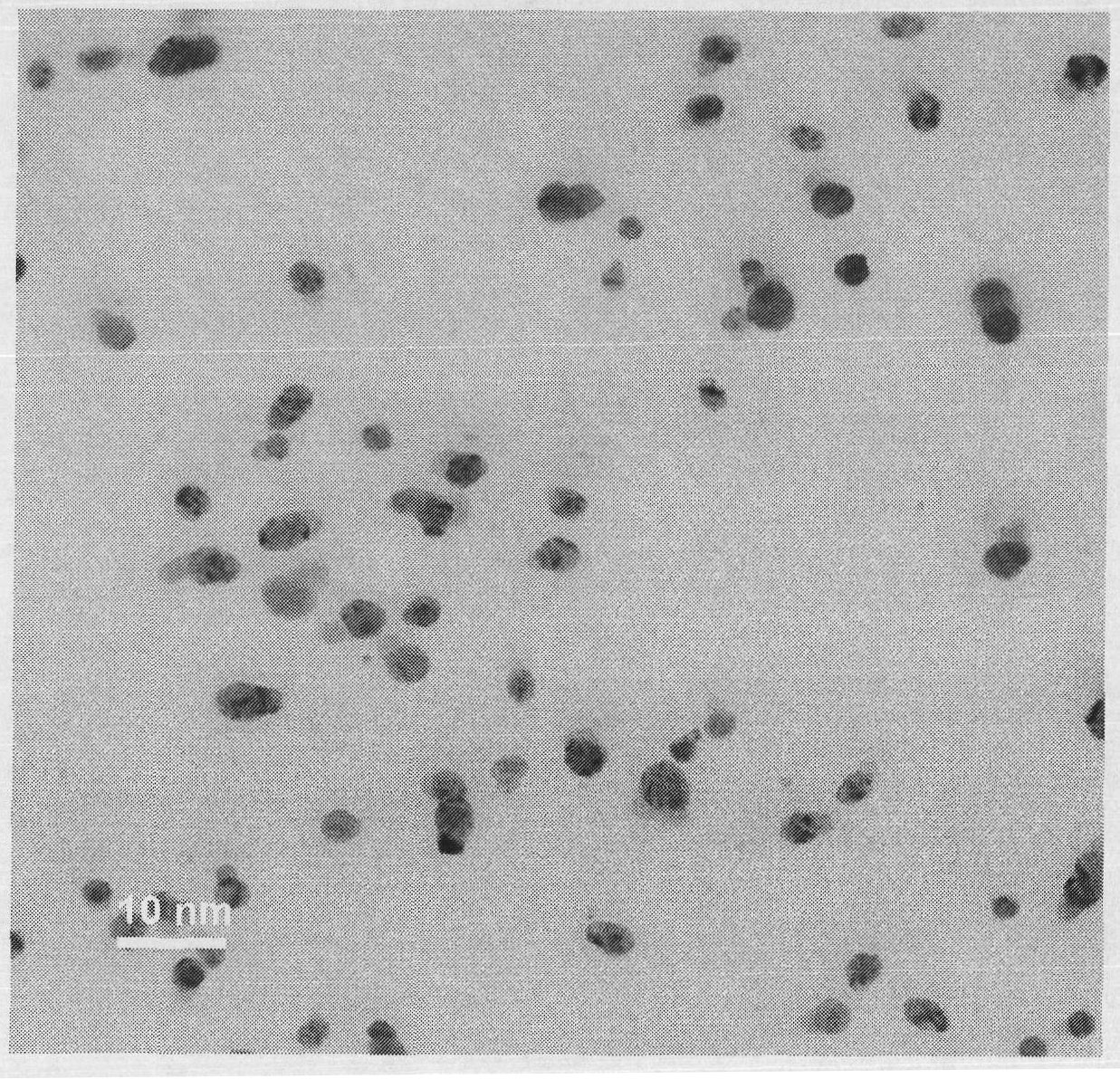
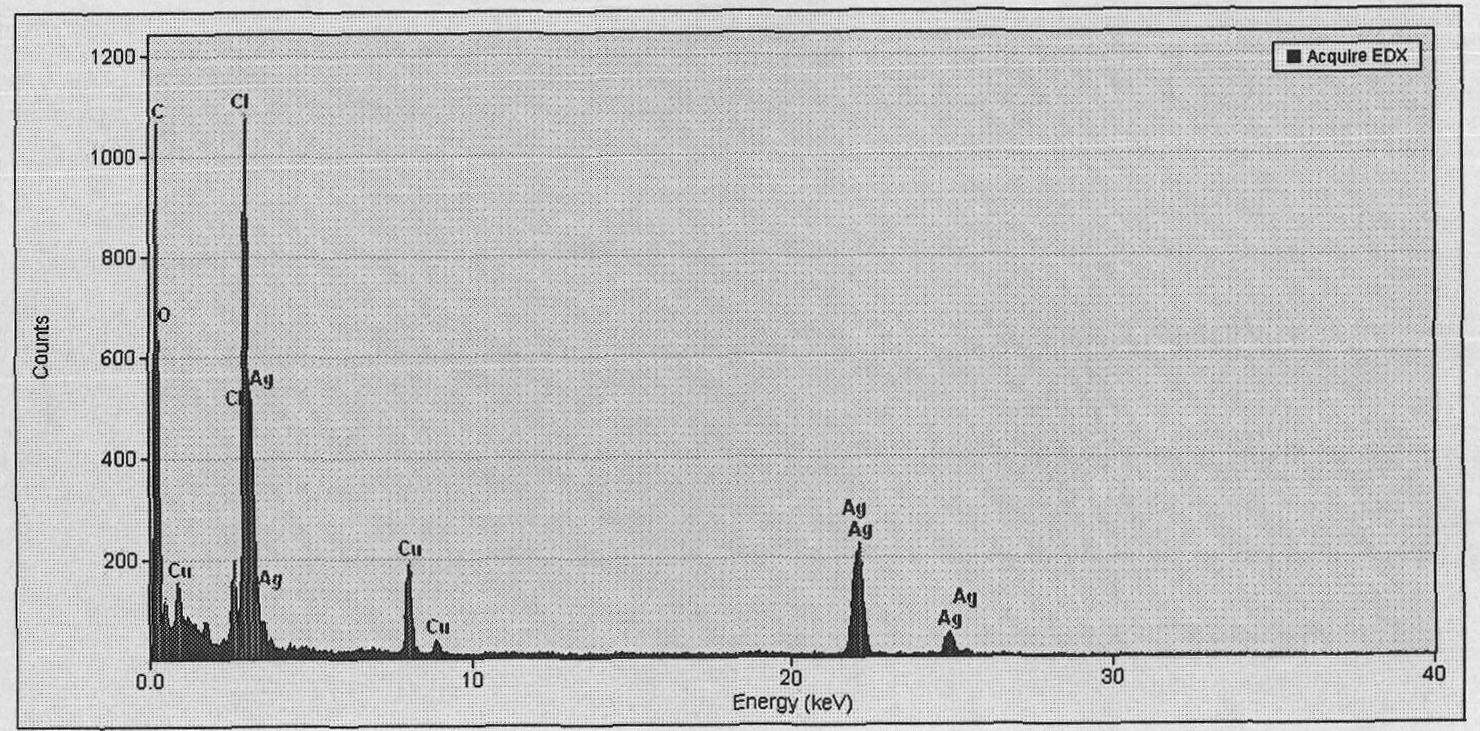
[0017] Step 2): Add 0.07 g of sodium chloride to the silver ion-containing hyperbranched polyglycidol aqueous solution obtained in step 1), and stir for 1 hr to obtain water-soluble silver chloride nanoparticles. The transmission electron microscope picture is attached figure 1 As shown, the particle size is 2-8 nanometers, and the particle dispersibility is good. The energy spectrum analysis diagram is attached figure 2 As shown, the presence of silver and chlorine is confirmed.
Embodiment 2
[0019] Step 1): Add 10 g of hyperbranched polyglycidol with a molecular weight of 80,000 to a flask containing 300 mL of deionized water. After stirring for 1 hour, add 0.5 g of silver nitrate and continue stirring for 2 hours to obtain a hyperbranched polyglycidol containing silver ions. Aqueous glycidol solution.
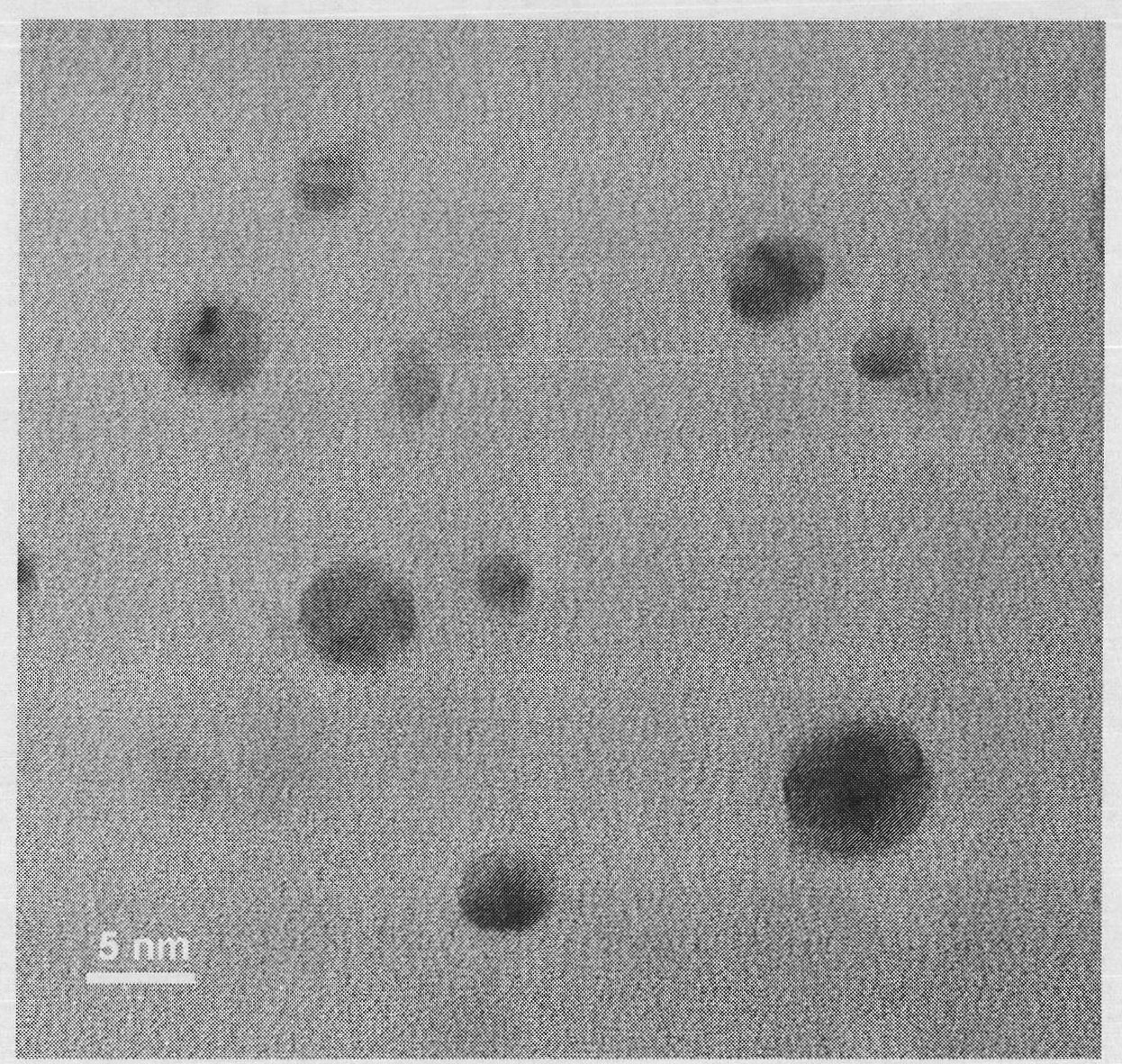
[0020] Step 2): Add 0.36 g of potassium bromide to the silver ion-containing hyperbranched polyglycidol aqueous solution obtained in step 1), and stir for 1 hr to obtain water-soluble silver bromide nanoparticles. The transmission electron microscope picture is attached image 3 As shown, the particle size is 2-6 nanometers, and the particle dispersibility is good. The energy spectrum analysis diagram is attached Figure 4 As shown, the presence of silver and bromine is confirmed.
Embodiment 3
[0022] Step 1): Add 2g of hyperbranched polyglycidol with a molecular weight of 10,000 to a flask containing 100mL of deionized water, and after stirring for 30 minutes, add 0.01g of silver sulfate and continue stirring for 2hr to obtain a hyperbranched polyglycidol containing silver ions. Aqueous glycidol solution.
[0023] Step 2): Add 0.0047 g of potassium chloride to the silver ion-containing hyperbranched polyglycidol aqueous solution obtained in step 1), and stir for 15 minutes to obtain water-soluble silver chloride nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com