High-activity cubic-block silver chloride micron-crystal and electrochemical preparation method thereof
A technology of silver chloride and cubes, applied in chemical instruments and methods, silver halide, chemical/physical processes, etc., can solve problems such as complicated experimental methods, and achieve simple synthesis methods and steps, good uniformity, and high crystallinity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Measure 100ml of deionized water into the beaker, add 1.42g of sodium sulfate to the above solution, stir for 10 minutes to fully dissolve the solid; then add 0.175g of sodium chloride solution into the above solution, and keep stirring to make the solution uniform Add the above solution into the H-type electrochemical reaction cell; finally put the 1×1cm silver sheet into one section of the reaction cell and connect it with the anode of the constant potential power supply, put the same size platinum sheet at the other end The cathode of the potential power supply is connected, the distance between the two electrodes is kept at 6 cm, the output voltage of the constant potential power supply is adjusted to 6V, and the reaction is carried out at room temperature for 30 minutes. Washed twice with deionized water, dried in a constant temperature oven at 60°C to obtain cubic silver chloride micro-crystals.
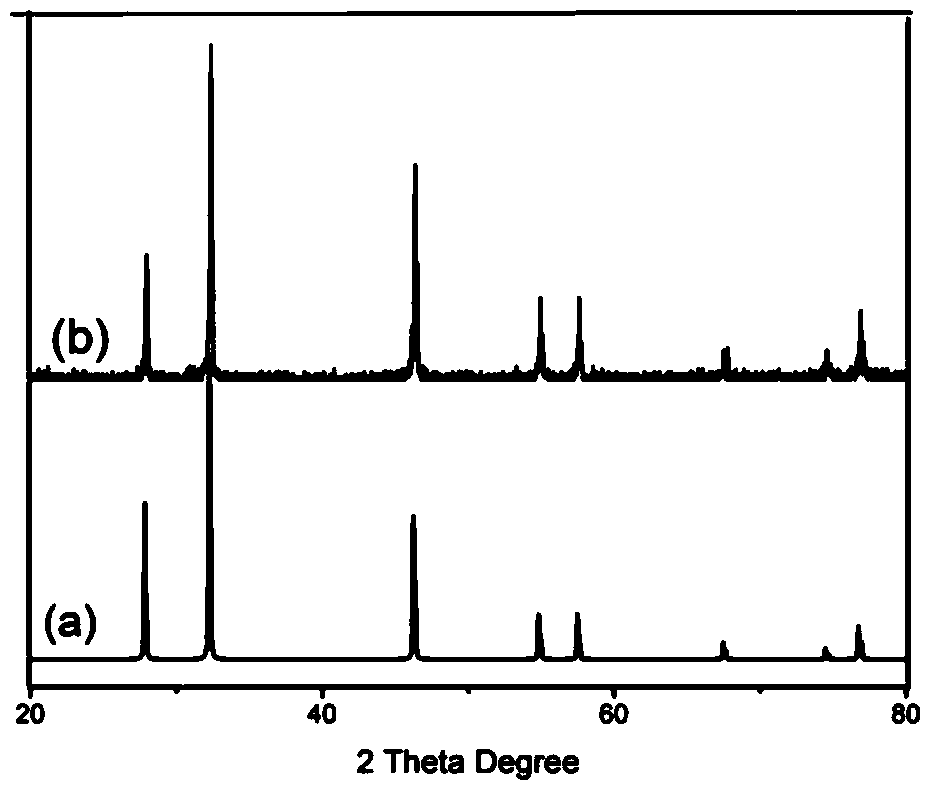
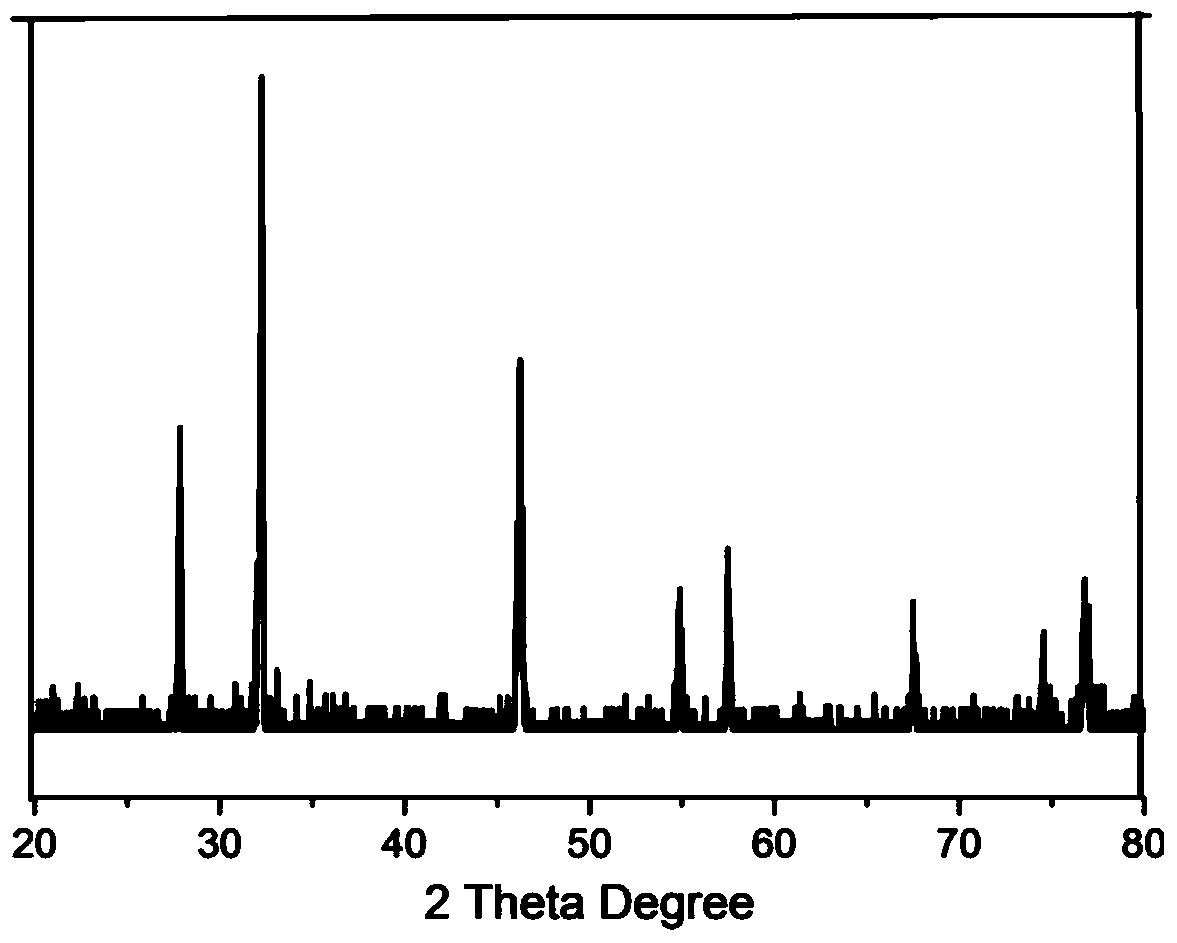
[0034] attached figure 1 (b) is the X-ray diffraction figure of em...
Embodiment 2
[0037] Measure 100ml of deionized water into the beaker, add 1.42g of sodium sulfate to the above solution, stir for 10 minutes to fully dissolve the solid; then add 0.175g of sodium chloride solution into the above solution, and keep stirring to make the solution uniform Add the above solution into the H-type electrochemical reaction cell; finally put the 1×1cm silver sheet into one section of the reaction cell and connect it with the anode of the constant potential power supply, put the same size platinum sheet at the other end The cathode of the potential power supply is connected, and the distance between the two electrodes is kept at 6 cm. The output voltage of the constant potential power supply is adjusted to 1.5V and reacted at room temperature for 30 minutes. The precipitation in the H-type electrochemical reaction cell on one side of the silver sheet is filtered. Wash twice with deionized water, and dry in a constant temperature oven at 60° C. to obtain cubic silver c...
Embodiment 3
[0040]Measure 100ml of deionized water into the beaker, add 1.42g of sodium sulfate into the above solution, stir for 10 minutes to fully dissolve the solid; then add 0.058g of sodium chloride solution into the above solution, and keep stirring to make the solution uniform Add the above solution into the H-type electrochemical reaction cell; finally put the 1×1cm silver sheet into one section of the reaction cell and connect it with the anode of the constant potential power supply, put the same size platinum sheet at the other end The cathode of the potential power supply is connected, and the distance between the two electrodes is kept at 6 cm. The output voltage of the constant potential power supply is adjusted to 3V and reacted at room temperature for 30 minutes. Washed twice with deionized water, dried in a constant temperature oven at 60°C to obtain cubic silver chloride micro-crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com