Method for the in vitro synthesis of short double stranded RNAs
A technology of RNA polymerase and polymerase, which is applied in the field of synthesizing short double-stranded target-specific RNAs, and can solve the problems that small interfering RNAs cannot be directly applied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
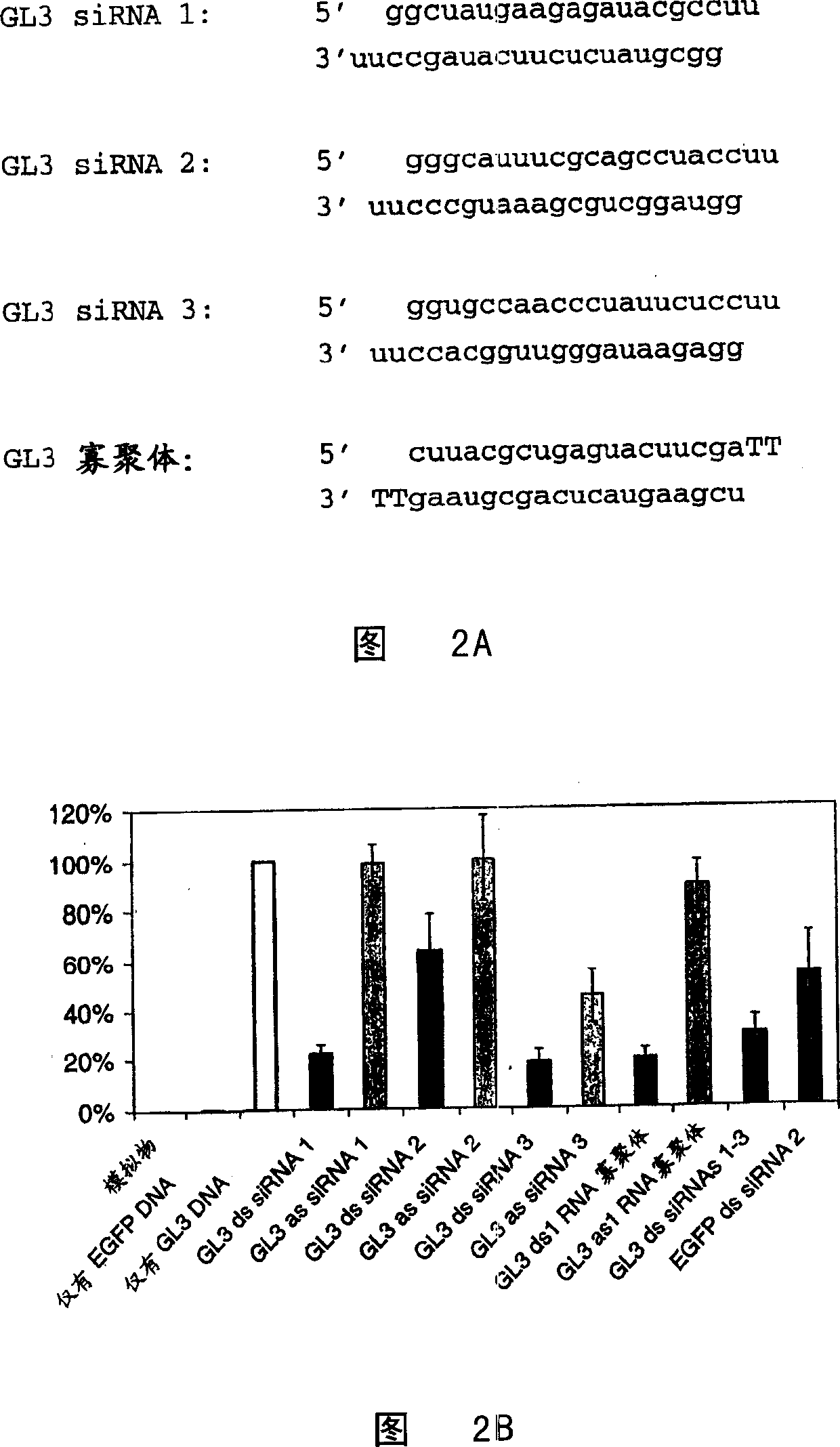
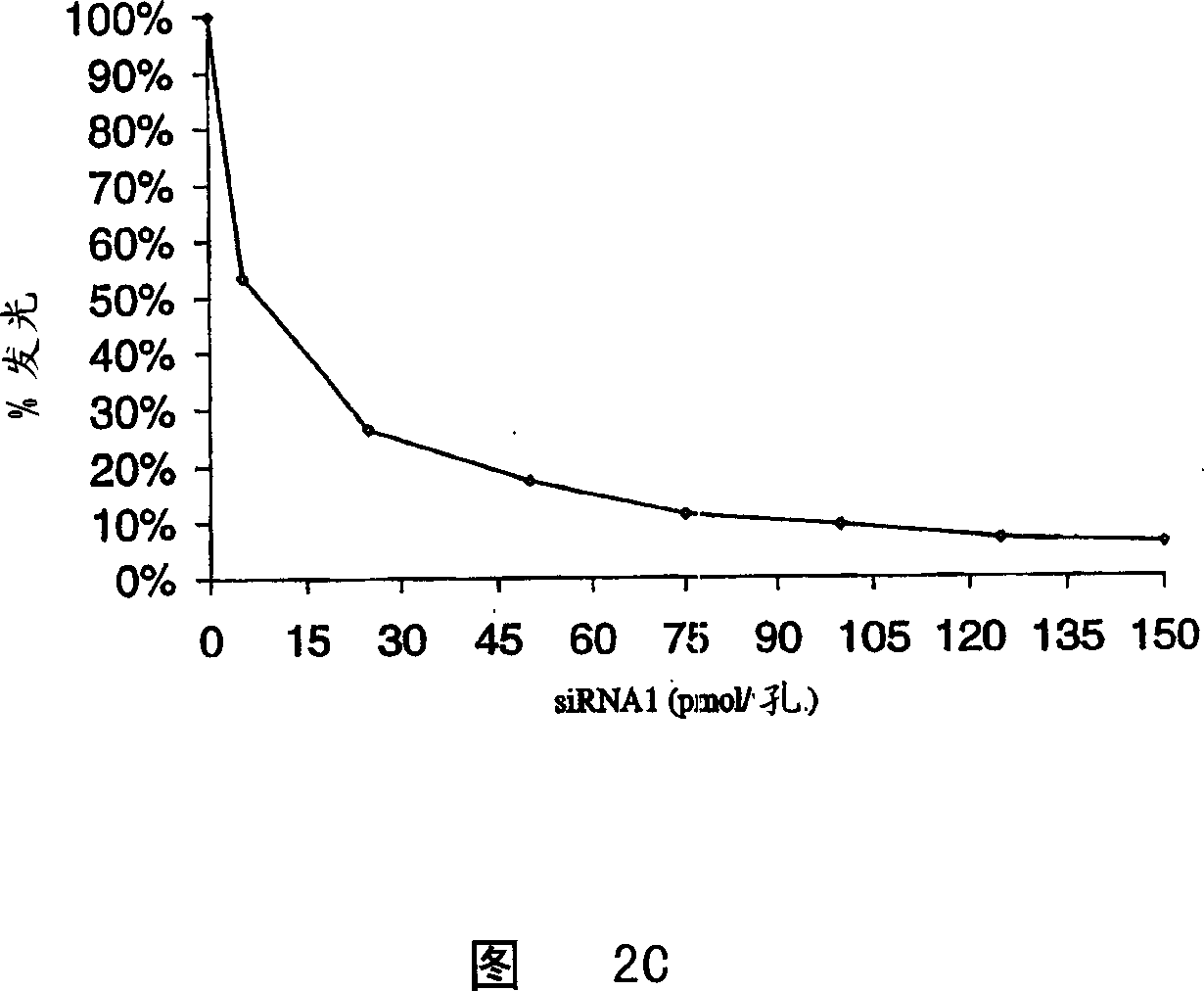
[0088] Example 1: EGFP and GL3-specific short dsRNAs transcribed in vitro, in human
[0089] Inducible RNA transfection in cell-like cells
[0090] Materials and methods
[0091] plasmid construct
[0092] Luciferase+ was expressed from the plasmid pGL3-control (Promega). EGFP is expressed by EGFP / pcDNA5-FRT, which includes the EGFP gene from pEGFP (Clontech), which is directionally linked to the HindIII and NotI sites of the mammalian expression vector pcDNA5 / FRT (Invitrogen).
[0093] In vitro transcription and hybridization of siRNAs
[0094] Hybridize the oligomer template strand to the sense T7 promoter sequence (5' TAATACGACTCACTATAGG) in 10 mM Tris-HCl pH 9.0, 100 mM NaCl, 1 mM EDTA, including boiling for 2 minutes and slowly cooling over 2-3 hours to room temperature.
[0095] Transcription is using MEGAshortscript TM T7 kit (Ambion), according to the manufacturer's instructions. siRNA was purified on G-25 spin columns. Phenol:chloroform:isoamyl...
Embodiment 2
[0116] Example 2: Mouse Insr-specific short dsRNAs transcribed in vitro, knocking out Balb / C
[0117] Insr in mouse liver
[0118] Male Balb / C mice (approximately 25 g) (standard housing, free access to food / water) received a tail vein injection with 2.3 ml of saline, or 40 μg of the mouse insulin receptor-targeted (NCBI deposit number NM-010568; 2536 -2556 bp) of siRNA prepared by the truncated T7 promoter method of in vitro transcription, while using 800 U RNase inhibitor.
[0119] The injections were performed as quickly as possible (8-10 seconds) and 2 control and 2 siRNA-treated mice were sacrificed at 24, 48 and 72 hours. The liver was quickly removed, weighed, and frozen in dry ice / isopropanol. Total RNA was extracted using crushed frozen tissue and the RNEasy Maxi kit (Qiagen).
[0120] After first-strand cDNA synthesis, insulin receptor mRNA was analyzed by Q-PCR using a Smart cycler (primers: F 3526-3548, R 3744-3768), and the results were also ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com