Quality control method for liuwei Dihuang soft extract
A quality control method, Liuwei Dihuang technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of catalpol instability when exposed to heat, difficulty in controlling the quality of Liuwei Dihuang paste, and large limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: Get 320g of Chinese herbal rehmannia glutinosa, 160g of Cornus officinalis (made), 160g of Chinese yam, 120g of Moutan cortex, 120g of Poria cocos, 120g of Alisma, add water to decoct the above-mentioned raw materials for three times, add 9 times the amount of water for the first and second times, Decoct for 2 hours, add 7 times the amount of water for the third time, decoct for 1 hour, combine the decoction, filter, let the filtrate stand, take the supernatant and concentrate it under reduced pressure to a clear liquid with a relative density of 1.28-1.32 (85°C). Ointment, add 300g of refined honey for every 100g of clear ointment, mix well, and get it.
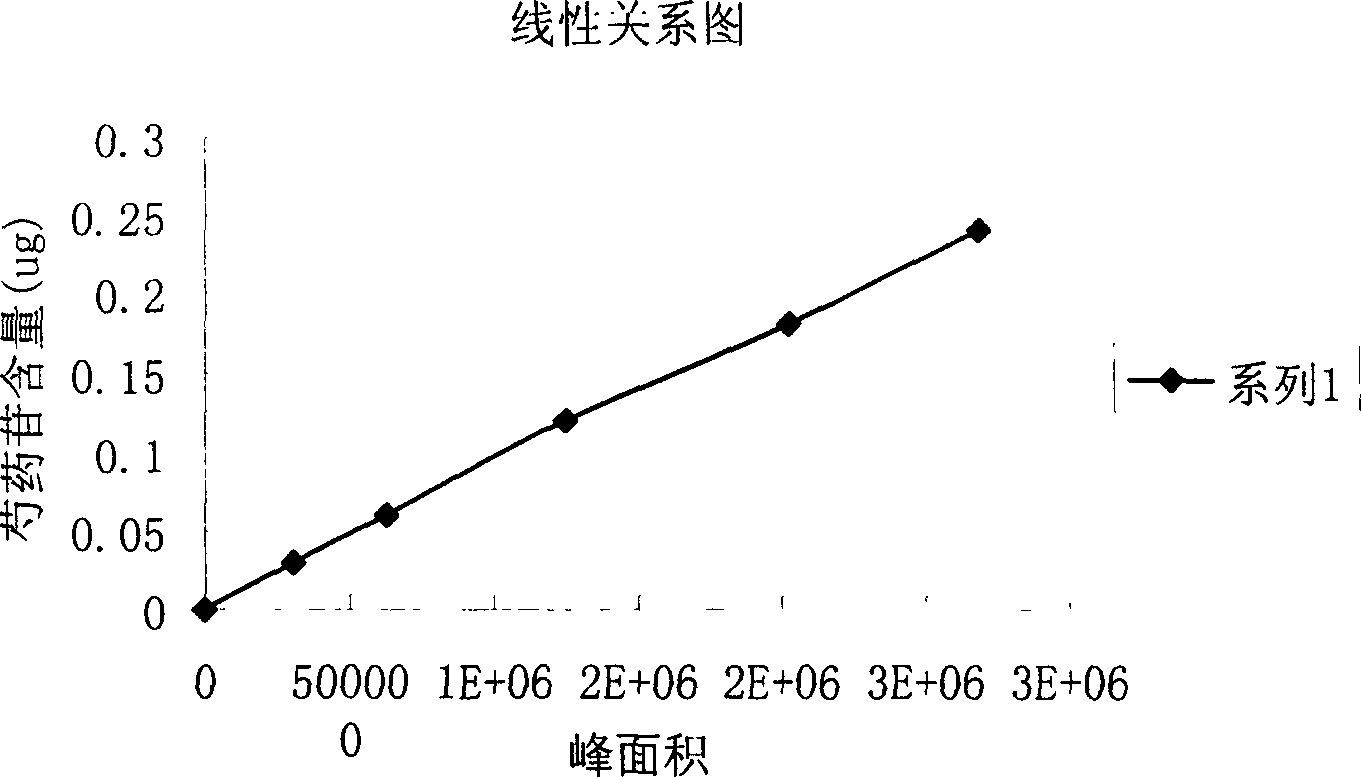
[0096] 1. Use high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition an appendix VID) to measure the content of paeoniflorin and its standards:
[0097] a. Chromatographic conditions: use octadecylsilane bonded silica gel as the filler; acetonitrile-water (12:88) as the mobile phase, ...
Embodiment 2
[0105] Embodiment 2: Get 320g of Chinese herbal medicine Rehmannia glutinosa, 160g of Cornus officinalis (made), 160g of Chinese yam, 120g of Moutan cortex, 120g of Poria cocos, 120g of Alisma, add water to decoct the above-mentioned raw materials for three times, add 9 times the amount of water for the first and second times, Decoct for 2 hours, add 7 times the amount of water for the third time, decoct for 1 hour, combine the decoction, filter, let the filtrate stand, take the supernatant and concentrate it under reduced pressure to a clear liquid with a relative density of 1.28-1.32 (85°C). paste. Add 300g of refined honey for every 100g of clear ointment, mix well, and you get it.
[0106] 1. Use high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition an appendix VID) to measure the content of paeoniflorin and its standards:
[0107] a. Chromatographic conditions: use octadecylsilane bonded silica gel as filler; acetonitrile-water (10:90) as mobile phas...
Embodiment 3
[0115] Embodiment 3: Get 320g of Chinese herbal rehmannia glutinosa, 160g of Cornus officinalis (made), 160g of Chinese yam, 120g of Moutan cortex, 120g of Poria cocos, 120g of Alisma, add water to decoct the above-mentioned raw materials for three times, add 9 times the amount of water for the first and second times, Decoct for 2 hours, add 7 times the amount of water for the third time, decoct for 1 hour, combine the decoction, filter, let the filtrate stand, take the supernatant and concentrate it under reduced pressure to a clear liquid with a relative density of 1.28-1.32 (85°C). paste. Add 300g of refined honey for every 100g of clear ointment, mix well, and you get it.
[0116] 1. Use high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition an appendix VID) to measure the content of paeoniflorin and its standards:
[0117] a. Chromatographic conditions: use octadecylsilane bonded silica gel as a filler; acetonitrile-water (15:85) is the mobile phase, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com