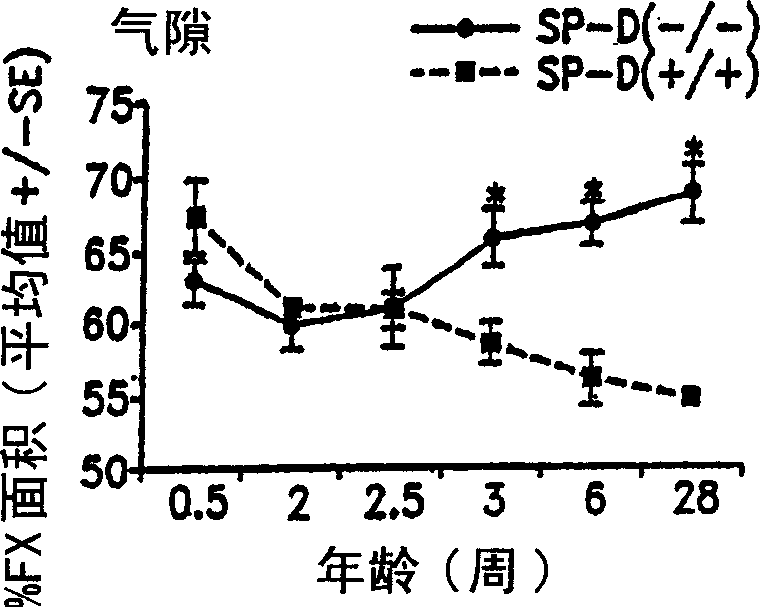
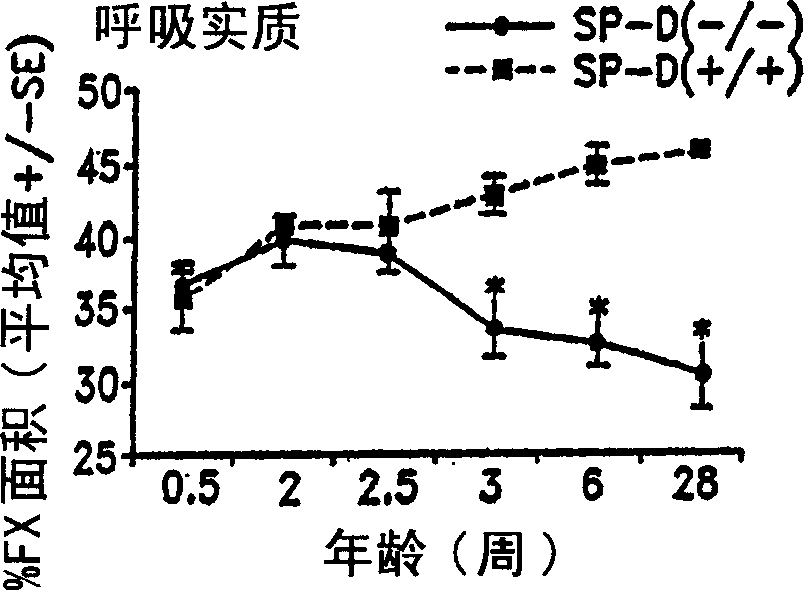
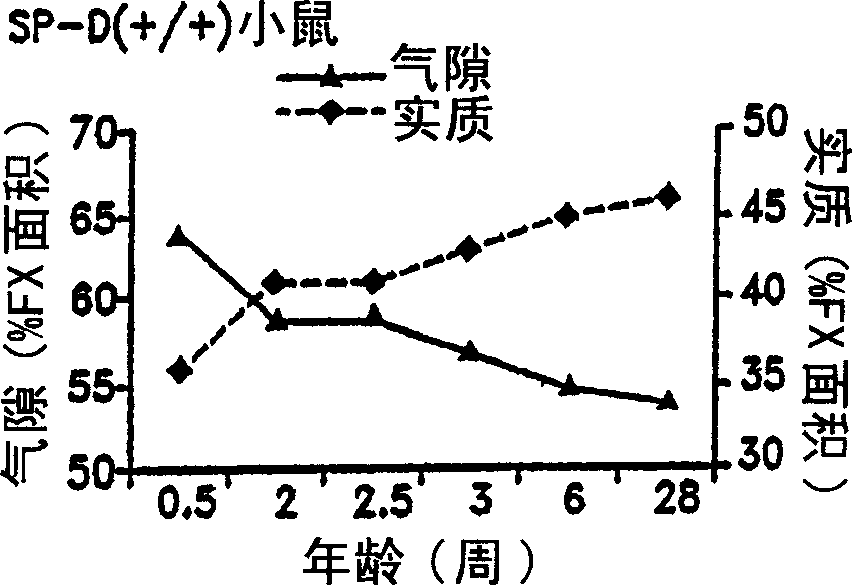
Surfactant protein for the prevention and diagnosis of pulmonary emphysema
An emphysema and active technology, applied in the field of biologically active proteins, can solve the problems of unidentified surfactant proteins, unclear effects, and unclear elaboration of SP-D
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Construction of SP-D(- / -) knockout mice
[0057] SP-D(- / -) mice were generated by targeted gene inactivation. Integration of the pGKneo targeting vector containing sequences from exon 2 of the SP-D gene produces a deletion of the second exon of the SP-D gene, which includes removal of the initiation methionine and translation initiation sequence. The sequence of exons 1 and 2 of the mouse SP-D gene can be found under Genebank accession number AF047741. The target was designed using pGKneo by first subcloning a 5.1-kb blunt-ended KpnI-tailed HindIII genomic fragment encoding intron 2 to exon 6 into the KpnI site between the neomycin resistance cassette and the thymidine kinase cassette to the carrier. Subsequently, an XhoI linker was attached to the tail of a 1.5-kb genomic PstI fragment containing part of intron I and cloned into the XhoI site 5' of the neomycin resistance cassette. Eight of the 104 ES clones subjected to the double selection process were correctly o...
Embodiment 2
[0064] Example 2 demonstrates the effect on phospholipid levels. Alveolar and tissue phospholipid levels, particularly phosphatidylcholine pool levels, were significantly increased, while total bronchoalveolar lavage (BAL) protein levels remained unchanged.
[0065] Example 2
[0066] Phospholipid levels in SP-D(- / -) mice
[0067] Alveolar, tissue and total saturated phosphatidylcholine (Sat-PC) (p3 H) Choline incorporation into whole lung Sat-PC is slightly increased 8 hours after injection, and incorporation is approximately 20% greater in SP-D(- / -) mice (p<0.05).
Embodiment 3
[0070] Decrease of SP-A level in SP-D(- / -) mice
[0071]No differences in SP-B and SP-C mRNA or protein were observed in SP-D(- / -) mice. In contrast, Northern hybridization and hybridization to the SP-A probe of total lung RNA from SP-D(+ / +), SP-D(+ / -), and SP-D(- / -) mice showed SP-A mRNA was reduced in SP-D(- / -) mice. Consistent with the reduction in SP-A mRNA, BAL SP-A protein was significantly reduced by approximately 25% in SP-D(- / -) mice as assessed by Western blot analysis of alveolar lavage from 3 mice.
[0072] Thus, SP-D plays a role in the regulation of SP-A production. Because SP-A is involved in host defense in the lung, SP-D can affect host defense in two ways. By upregulating SP-A production and by directly interacting with immune and microbial cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com