Cloning of goat ACE2 gene and preparation of mouse anti-goat polyclonal antibody
A technology of goats and genes, applied in genetic engineering, plant genetic improvement, anti-enzyme immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 Amplification of ACE2 gene, and construction of connection vector
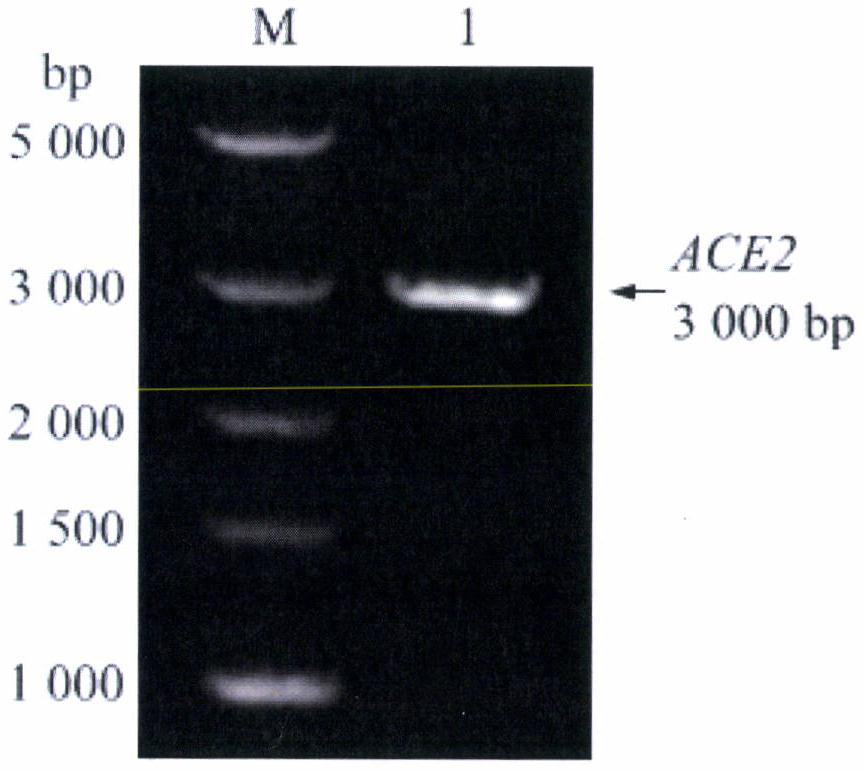
[0044] Primers were designed according to the bovine ACE2 gene sequence (NM_001024502) in GenBank, and the upstream and downstream primers were inserted into the BamHI and HindIII restriction sites respectively, and the primers were synthesized by Nanjing Qingke Biotechnology Co., Ltd. Reverse transcribe RNA into cDNA, use cDNA as a template for PCR amplification, reaction system: cDNA template 2uL, reaction conditions: 95°C, 3min; 95°C, 10s; 59°C, 30s; 72°C, 1min30s; 35 cycles ; 72°C, 10 min. The results of PCR amplification were as figure 1 , a single band appeared (see lane 1), and the band size was about 3000bp, which was consistent with the expected (2954bp) size.
[0045] Purify and recover the PCR product using the DNA gel recovery kit, and refer to the instructions of the DNA gel recovery kit for specific steps. The recovered product was ligated with the pMD19T vector (16°C, over...
Embodiment 2
[0049] Expression experiment of embodiment 2 protein
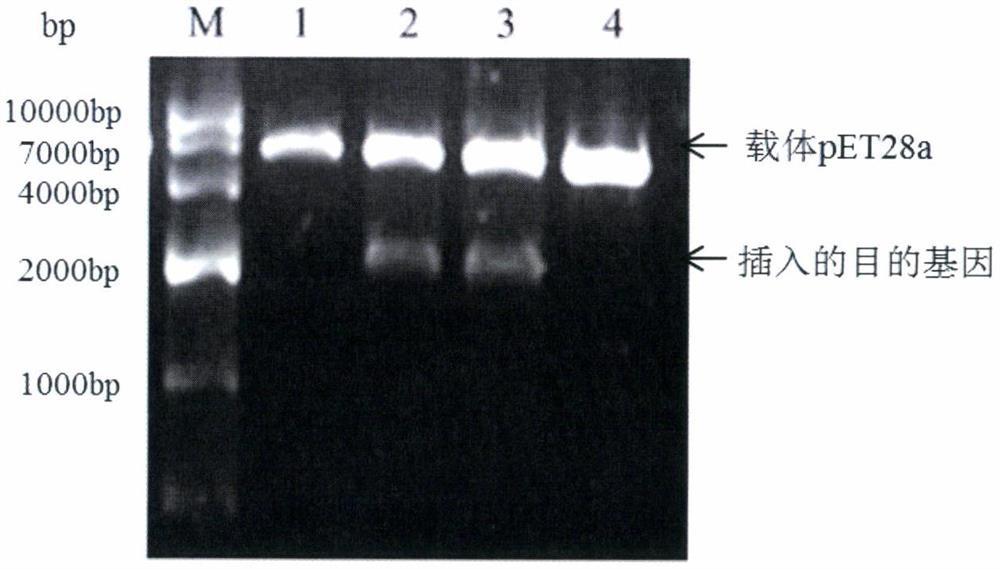
[0050] The correct pMD19T-ACE2 recombinant plasmid and pET32a prokaryotic expression vector were identified by sequencing using HindIII / BamHI double enzyme digestion, and the results of pET28a plasmid double enzyme digestion are shown in the figure Figure 3A , The pET28a plasmid was verified by double digestion with BamHI and HindIII, and the digested product was identified by 1% agarose gel electrophoresis, and specific bands appeared at 5kb and 2kb, which proved that the digestion was successful. The result of double enzyme digestion of pET32a-ACE2 recombinant plasmid is as follows: Figure 4 , pET32a-ACE2 recombinant plasmid BamHI, HindIII double enzyme digestion showed that specific bands appeared at 6kb and 2kb, that is, the size of the vector pET32a and the inserted target gene ACE2 was consistent with the size of the vector and the target fragment, proving that the enzyme digestion was successful.
[0051] The rec...
Embodiment 3
[0054] Example 3 protein purification experiment
[0055] Prepare 10% separating gel and 5% stacking gel without inserting a comb. After the gel is solidified, add 500uL of inclusion body extract treated with loading buffer, and prepare the gel according to the conventional method, and add standard molecular weight protein as a reference. SDS-PAGE was carried out by conventional methods.
[0056]After electrophoresis, put the unloaded gel into a clean large plate, and perform 0.25mol / L KCl staining on a shaker for 10 minutes. After staining, cut off the silver-white stained target band with a scalpel, and wash 5 times with distilled water. Finally, transfer the gel into a clean pouch, add 500uLPBS to shake and mix, freeze and thaw three times at -20°C, 12000r / min, centrifuge for 3min, take the supernatant for SDS-PAGE electrophoresis, and check the purity by Western blot. Specific operation of Western blot identification of ACE2 recombinant protein: after SDS-PAGE electrophor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com