A method for treating allergies
An allergic, atopic technique for use in the field of cathepsin S inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Cathepsin S inhibition test.
[0160] Recombinant human cathepsin S (CatS) was expressed in a baculovirus system and purified in one step using a propylthio-dextran column. 10 L yielded about 700 mg of CatS, and the N-terminal sequence was characterized. The assay was performed in 100 mM sodium acetate pH 5.0 containing 1 mM DTT and 100 mM NaCl. The substrate of the test is
[0161] (Aedens) EKARVLAEAA (Dabcyl) K-amide
[0162] The substrate Km is about 5 uM, but the presence of substrate inhibition makes kinetic analysis difficult. For 20 uM substrate, the assay speed was linear for the range of 1-8 ng of CatS in a 100 μl reaction and gave an approximately 7-fold signal after 20 minutes consuming only 20% of the substrate. The main assay was started after stopping the reaction with 0.1% SDS and fluorescence was subsequently measured. For other tests, check every 20 minutes. The rate was calculated from the increase in slope and from this the percent inhibition wa...
Embodiment 2
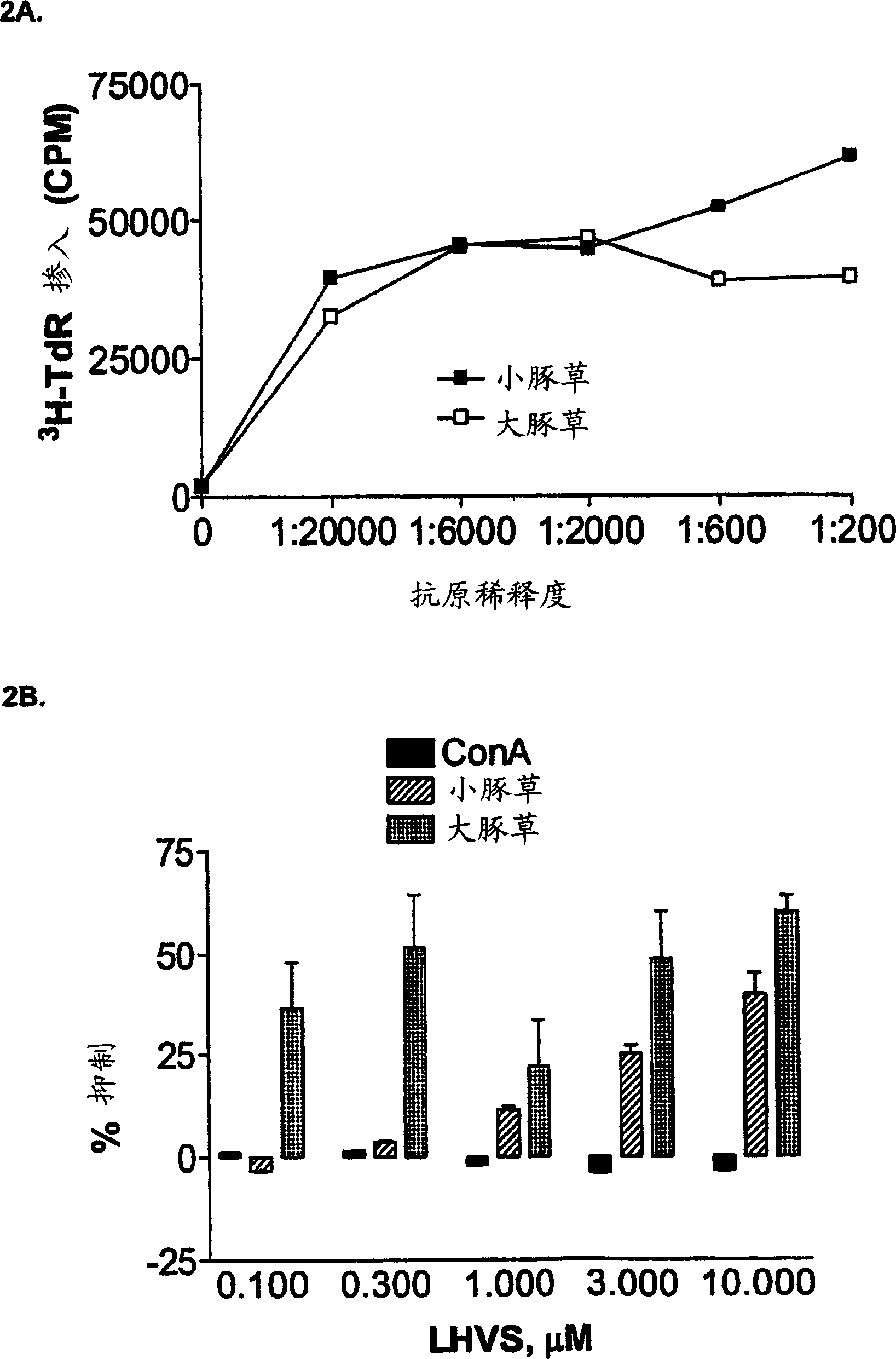
[0164] Ex Vivo Inhibition of Allergic Responses by Cathepsin S Inhibitors
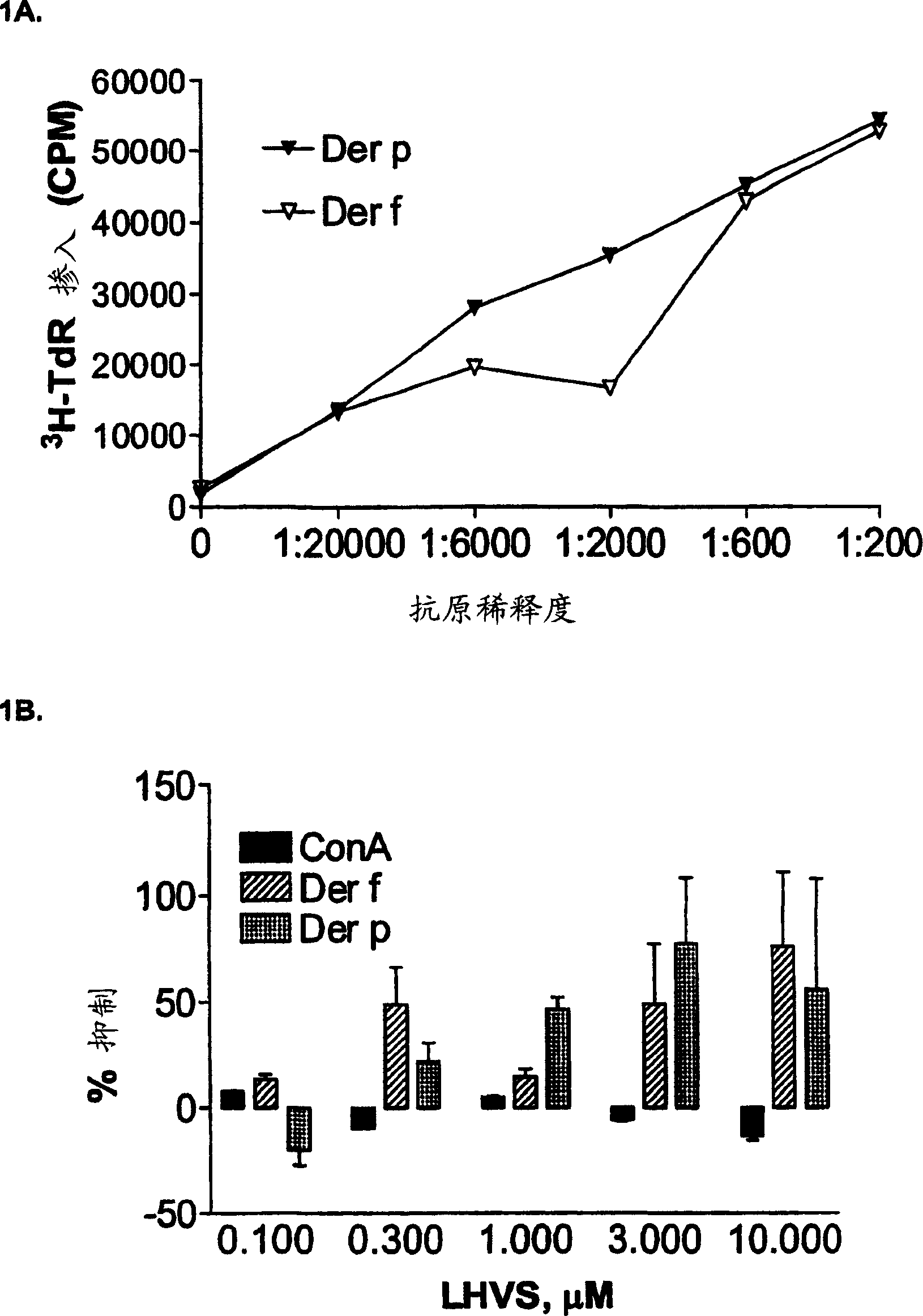
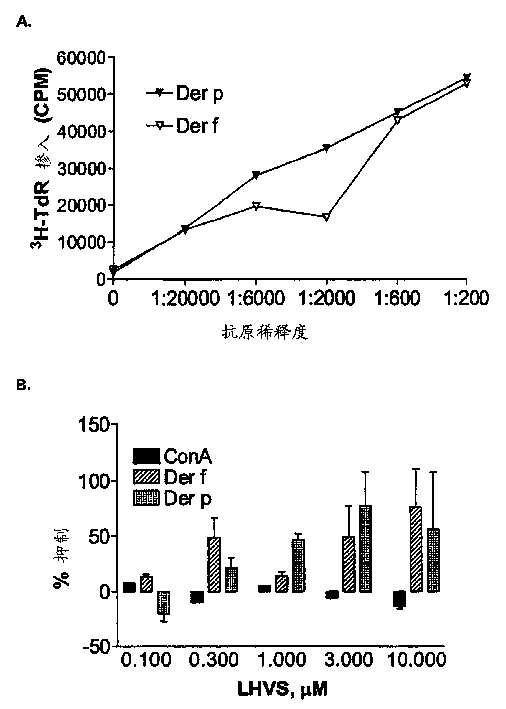
[0165] The following experiments demonstrate that cathepsin S inhibitors block human T cell responses to crude allergen extracts.
[0166] Materials and methods
[0167] Reagents: Glycerated crude allergen extracts of D. farinae (Dermataphagoides pteronyssinus, Dermataphagoides farinae) and ragweed [Ambrosia trifida (large), Ambrosia artemisiifolia (small)] were purchased from Hollister-Stier Laboratories (Minneapolis, MN). Concanavalin A (ConA) was purchased from Calbiochem (La Jolla, CA).
[0168] Donors: All allergic donors were screened for specific allergens using the RAST test. The HLA II haplotypes of these donors were determined by PCR.
[0169] Cell Culture: Human peripheral blood mononuclear cells (PBMC) were purified from Ficoll-Hypaque gradients of blood from allergic donors, followed by washing with phosphate-buffered saline (PBS). PBMC at 0.5-1.0×10 6 Concentrations of cells / well wer...
Embodiment 3
[0179] Monitoring Cathepsin S Inhibition in Human Blood
[0180] The effect of in vivo administration of the cathepsin S inhibitor in the clinical test kit can be monitored by detecting the accumulation of the rapid degradation product of the invariant chain (li), that is, the p10li fragment, in the blood of the subject. After administration of a cathepsin inhibitor for a period of time, e.g., 0.01 to 50 mg / kg / day, resulting in a plasma concentration of preferably 1 nM to 10 μM at 16 to 30 hours, blood is drawn and leukocytes are separated, e.g., by lysing red blood cells or by Ficoll-Hypaque Gradient centrifugation. Whole cell lysates of WBC were then prepared and analyzed by Western blot assay or ELISA assay. For Western blot experiments, cell lysates were first resuspended on SDS-PAGE gels. After transfer to nitrocellulose membranes, li and intermediate degradation products, including p10li, can be detected with a mouse mAb against li, such as Pin1.1, or with a rabbit pol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com