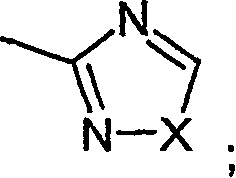
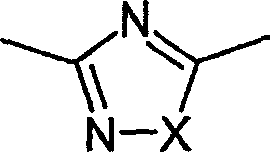
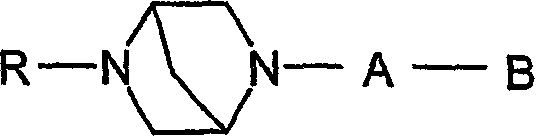Novel diazabicyclic biaryl derivatives
A technology for diazabicyclic and derivatives, which is applied in the field of diazabicyclic biaryl derivatives, and can solve problems such as not being disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Preparation Example
[0199] All reactions involving air-sensitive reagents or intermediates were performed under nitrogen and anhydrous reagents. Magnesium sulfate was used as drying agent in the work-up procedure and the solvent was evaporated under reduced pressure.
[0200] 2-[6-Bromo-3-pyridazinyl]-(1S,4S)-5-tert-butoxycarbonyl-2,5-diazabicyclo -[2.2.1]-Heptane (intermediate compound)
[0201] Tert-butyl-(1S, 4S)-(+)-2,5-diazabicyclo-[2.2.1]-heptane-2-carboxylate (3.0g, 15.1mmol), 3,6 - A mixture of dibromopyridazine (3.6 g, 15.1 mmol) and dioxane (15 ml) was stirred at 90° C. for 3 days. The crude salt was filtered. Aqueous sodium hydroxide solution (50ml, 1M) was added to the solid material, and the mixture was extracted with dichloromethane. Chromatography on silica gel with dichloromethane, methanol and concentrated aqueous ammonia (89:10:1) gave the title compound as the free base. Yield 1.71 g (32%).
[0202] 2-[6-(3-Thienyl)-3-pyridazinyl]-(1S,4S...
Embodiment 2
[0234] rat brain 3 Inhibition of H-α-bungarotoxin binding in vitro
[0235] In this example, the alpha of the compounds of the invention on the nicotinic receptor 7 -Affinity for isoform binding.
[0236] α-Bungarotoxin is a peptide isolated from the venom of the Cobra snake Bungara. It has high affinity for neuronal and neuromuscular nicotinic receptors, where it acts as a potent antagonist.
[0237] alpha found in the brain 7 Subunit isoforms and alpha in the neuromuscular junction 1 isotype formation 3 H-α-bungarotoxin labels nicotinic acetylcholine receptors.
[0238] tissue preparation
[0239] Prepare at 0-4°C. Use an Ultra-Turrax homogenizer in 15 ml of 118 mM NaCl, 4.8 mM KC1, 1.2 mM MgSO 4 and 2.5mM CaCl 2 Cerebral cortices from male Wistar rats (150-250 g) were homogenized in 20 mM Hepes buffer (pH 7.5) for 10 seconds. The tissue suspension was centrifuged at 27,000 xg for 10 minutes. The supernatant was discarded and the pellet was washed twice by cent...
Embodiment 3
[0248] in cortical synaptosomes 3 Inhibition of H-5-Hydroxytryptamine Uptake in Vitro
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com