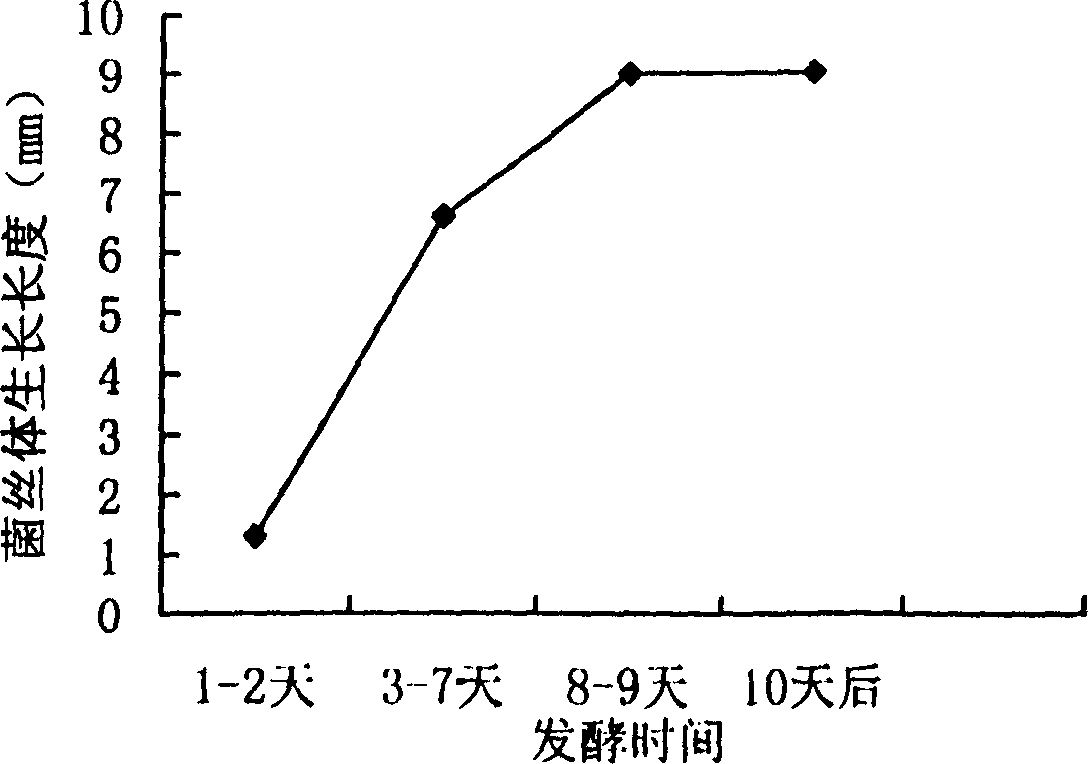
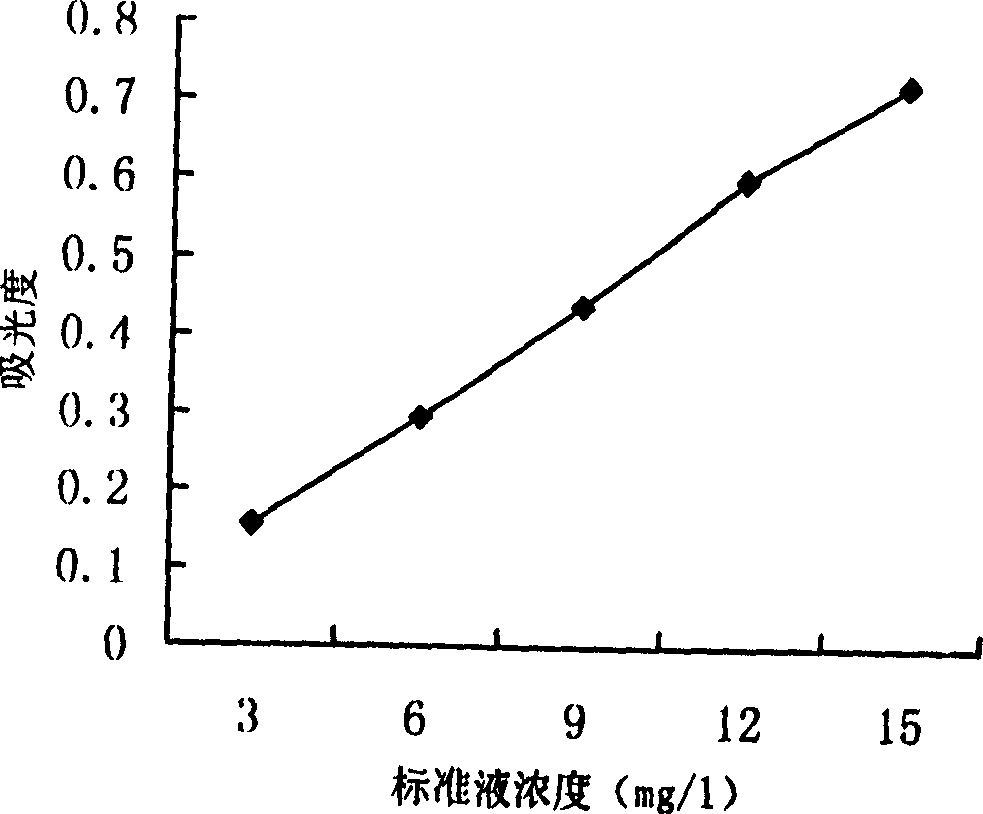
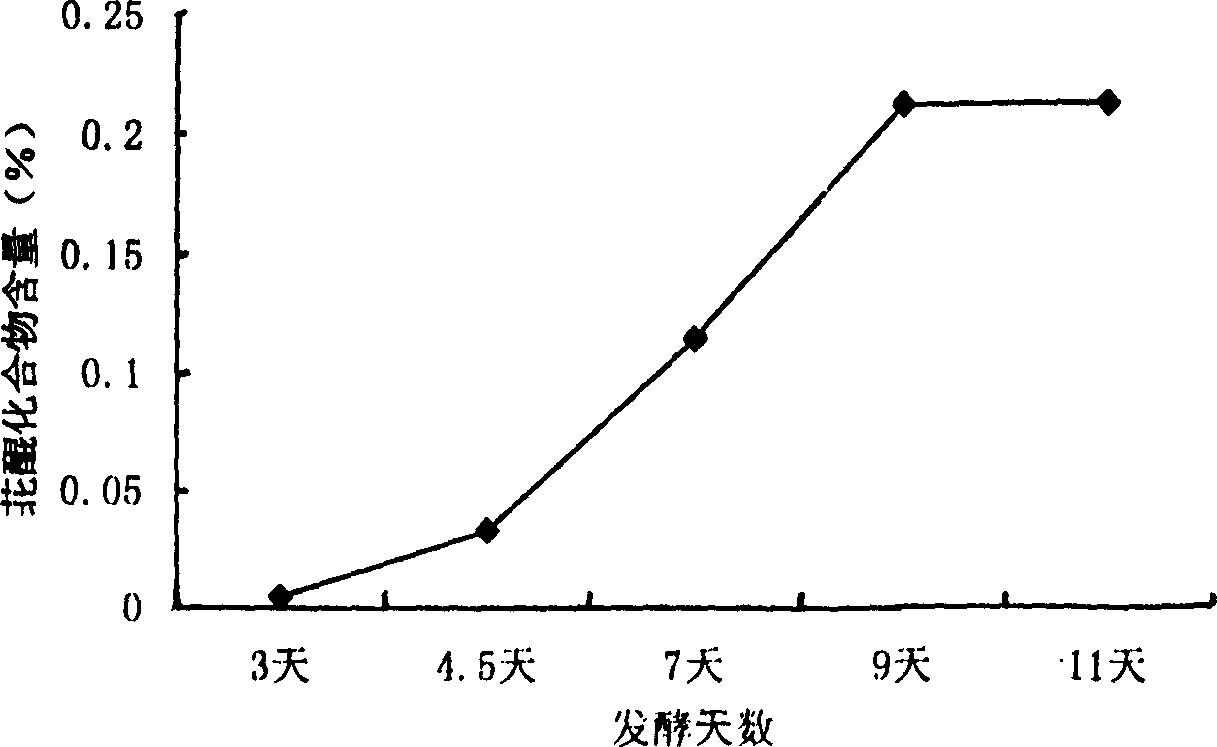
Biologic synthesis of perylene quinone compound
A biosynthesis and compound technology, applied in the field of biocatalytic synthesis of compounds, can solve the problems of cumbersome artificial synthesis steps and low total amount of perylenequinone compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0063] See the examples below.
[0064] The fungus Ascomycetes Hypomyces(Fr)Tul.sp, which grows in the mountainous area of western Yunnan, is identified as Ascomycetes, Hypomycetes, and mycoparasites after collection. The slant medium was potato juice dextrose agar medium. The seed medium was 20 grams of glucose, 1 gram of KH per liter of potato juice 2 PO 4 , 0.5 g MgSO 4 .7H 2 O, 0.1 g CaCl 2 .2H 2 O, 10 mg Vitamin B 1 , adjusted to PH=6 with 0.1M NaOH, and sterilized at 120° C. for 30 minutes in a sterilizing pot. The solid base medium is m (corn flour): m (wheat bran): m (okara): 1:1:1, 1‰ peptone, adding appropriate amount of bagasse, the ratio of material to water is 10:3, and sterilized.
[0065] Seed culture: Add 150 ml of seed culture medium to a 500 ml Erlenmeyer flask, sterilize with steam at 120°C for 30 minutes, insert the strain after cooling, and place on a compound shaker at 120 rpm at a constant temperature of 26°C Shake continuously for 4 days to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com